2016 Volume 56 Issue 11 Pages 2062-2067
2016 Volume 56 Issue 11 Pages 2062-2067
Precipitation behavior of copper sulfides in Fe-3.2%Si-0.4%Cu-0.02%S (mass%) ferritic steel was studied by X-ray diffraction (XRD), microstructure examination by transmission electron microscope (TEM) and scanning electron microscope (SEM). From the TEM results, it was found that FeS and cubic Cu2-xS, precipitated in as-cast specimens. XRD peaks due to the cubic Cu2-xS almost disappeared at 1173 K. XRD peaks due to the hexagonal Cu2-xS were identified in specimens annealed above 1373 K. Assuming that the hexagonal Cu2-xS is metastable in steel, which precipitates during cooling after dissolution of the stable cubic Cu2-xS, it can be suggested that the cubic Cu2-xS was dissolved at least 1173 K. Results of thermodynamic calculations are consistent with this suggestion, which indicates that the dissolution temperature of the cubic Cu2-xS is lower than those of other sulfides such as MnS and FeS in the ferritic steel.
Various kinds of sulfides, such as MnS,1,2,3) FeS4,5) and Cu2-xS (x=0−0.2),6,7,8) significantly influence the grain growth9,10,11,12) and mechanical properties13,14,15) of steel materials. Because Cu2-xS finely precipitates with diameter of 20–100 nm,16,17,18,19) the grain growth of steel is expected to be effectively retarded by Cu2-xS according to Zener’s formula. Although more information about precipitation and dissolution behaviors of the Cu2-xS are necessary to control the grain growth and mechanical properties of steel materials, no consensus has been achieved for the dissolution temperature of the Cu2-xS.
For example, Sakai et al.11) proposed a value of 1473 K for the dissolution temperature of the Cu2-xS in a 3%Si-added steel with 0.05%Cu and 0.02%S based on the chemical analysis. They performed the chemical analysis on the heat treated samples followed by water quenching. Also, the solubility product of the Cu2-xS in ferritic steel was obtained by Shimazu et al.16) as
| (1) |
The solubility product suggests that the dissolution temperature of the Cu2-xS (0.05%Cu and 0.02%S) is about 1596 K. Although they do not report the experimental details on how to obtain the solubility product in the literature, their results are relatively consistent with the experimental results reported by Sakai et al.11)
On the other hand, from the transmission electron microscope (TEM) observation, Ishiguro et al.19) implied that the Cu2-xS in low-carbon steel with 0.01%Si, 0.01%Cu and 0.007%S dissolved below 1023 K. They precisely investigated the precipitated form of Cu2-xS by TEM observation and concluded that Cu2-xS is precipitated even during a short time in water quenching. We consider that the complex precipitation behavior of the Cu2-xS in steel may cause the misinterpretation on the dissolution temperature of Cu2-xS in steels. That is, from the chemical analysis only, one would estimate the dissolution temperature of Cu2-xS in steels to be higher, because Cu2-xS are precipitated after heat treatment as pointed out by Ishiguro et al.19)
Qi et al. estimated the solubility product of the Cu2-xS in the Fe–Cu–S ternary ferritic steel as
| (2) |
There are some researchers who suggest that the Cu2-xS is thermodynamically stable11,16) and the dissolution temperature of the Cu2-xS is close to that of the MnS.22) On the other hand, others19,20,21) suggest that the solubility product of the Cu2-xS is larger than that of MnS, i.e., the dissolution temperature of the Cu2-xS should be lower than that of MnS.
We consider that such a contradiction is caused by the complex precipitation behavior of the Cu2-xS in steel.In the present work, in order to solve the above controversies and determine the dissolution temperature of the Cu2-xS in steel, experimental investigation of the precipitation behavior of the Cu2-xS in Fe-3.2%Si-0.4%Cu-0.02%S ferritic steel was carried out.
Cu-added ferritic steel, whose chemical composition is Fe-3.2%Si-0.4%Cu-0.02%S (mass%), was prepared from electrolytic Fe by vacuum induction melting. Small specimens (100×50×10 mm3) for heat-treatment were taken from an as-cast ingot (370×110×110 mm3). In order to avoid the segregation of Cu, the small specimens were cut from columnar crystal parts of the ingot. Chemical composition of the small specimens was confirmed to be Fe-3.2%Si-0.4%Cu-0.02%S. The Si and Cu contents were determined by the optical emission spectroscopy, while the S content was determined by the gas infrared absorption method after combustion in an induction furnace.
Each specimen was heat-treated at a particular temperature between 973 K, 1173 K, 1373 K and 1613 K for 1500 s under an Ar gas atmosphere, followed by quenching into iced water. The cooling rate was measured to be about 70 K/s at the central part of the specimens. Samples (15×15×7 mm3) for measurements were taken from inner parts of the heat-treated specimens. The heat-treated sample at 1613 K was reheated at 973 K for 3600 s under Ar gas atmosphere and quenched into iced water.
Precipitates in the samples were observed by a field emission TEM (FE-TEM, JEM-2100F, JEOL) and a scanning electron microscopy (FE-SEM, JSM-7000F, JEOL). For the TEM observation, selective potentiostatic etching (−100 mV vs SCE) by electrolytic dissolution method23) in 10%-AA electrolytic solution was performed on the mirror-polished samples. Precipitated particles are picked up by the carbon extraction replica method (Ni mesh) for the TEM observation.. Chemical compositions of precipitates were analyzed by energy-dispersive X-ray spectroscopy (TEM-EDS). For the SEM observation, selective potentiostatic etching by electrolytic dissolution method23) in 10%-AA electrolytic solution was performed on the mirror-polished samples.
Precipitated particles were extracted by electrolytic method in 10%-AA electrolytic solution23) whose crystal structures were characterized by X-ray diffraction (XRD) method. The XRD measurements were performed by an X-ray powder diffractometer (RINT1500, RIGAKU) with Cu Kα radiation.
Figure 1 shows TEM images and EDS spectra of the precipitates in an as-cast sample, i.e., non-heat-treated sample. Coarse spherical precipitates and rectangular precipitates were observed, as shown in Figs. 1(a)–1(e). In Figs. 1(f) and 1(g), the EDS spectrum of the precipitated particle contains strong peaks due to Fe and S elements, while that of the rectangular precipitate comes mainly from Cu and S elements. From the EDS results, we suggest that the precipitated particles are FeS and the rectangular precipitates are Cu2-xS.

TEM images [(a)–(e)] of precipitates in an as cast sample. A coarse spherical precipitate (d) and a rectangular precipitate (e). EDS spectra of a coarse spherical precipitate (f) and a rectangular precipitate (g). (Online version in color.)
Next, we discuss the crystal structures of the precipitates in Figs. 1(d) and 1(e), that is FeS and Cu2-xS. Figure 2 shows the electron diffraction patterns of Cu2-xS and FeS. According to the diffraction patterns, the crystal structures of Cu2-xS are identified to be cubic (JCPDS: 024-0061), while that of FeS is identified to be hexagonal (JCPDS: 037-0477). Detailed crystal structure of sulfides appeared in this study was summarized in Tables 1 and 2.
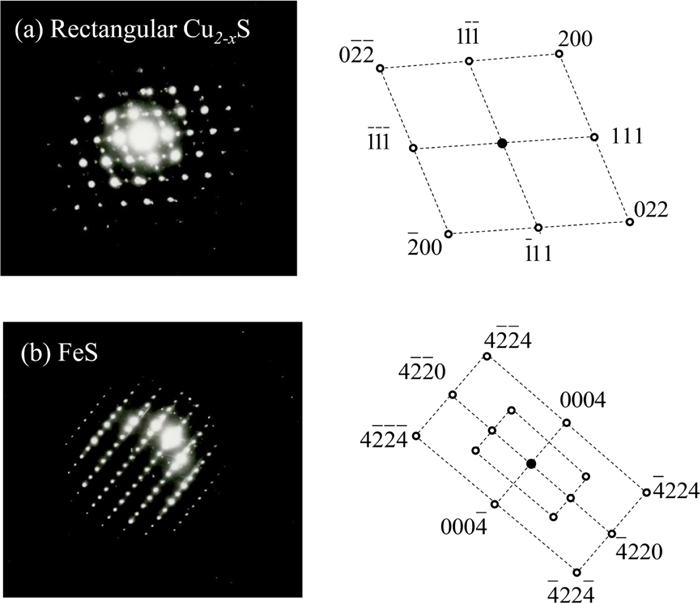
Electron diffraction patterns of rectangular Cu2-xS (a) and FeS (b) with zone axes [01-1] and [01-10] respectively. (Online version in color.)
| Sulfides | Structure type | Peason symbol | Space group |
|---|---|---|---|
| c-Cu2-xS | CaF2 | cF12 | Fm3m |
| h-Cu2-xS | InNi2 | hP6 | P63/mmc |
| FeS | NiAs | hP4 | P63/mmc |
Figure 3 shows SEM images of several precipitates in isothermally heat-treated and two-stage heat-treated specimens. Coarse spherical precipitates assigned to FeS are observed in samples heat-treated at 973 and 1173 K. One can notice that the precipitates are finely dispersed in samples heat-treated at above 1373 K and the heat-treated samples at 1613 K followed by reheating at 973 K.
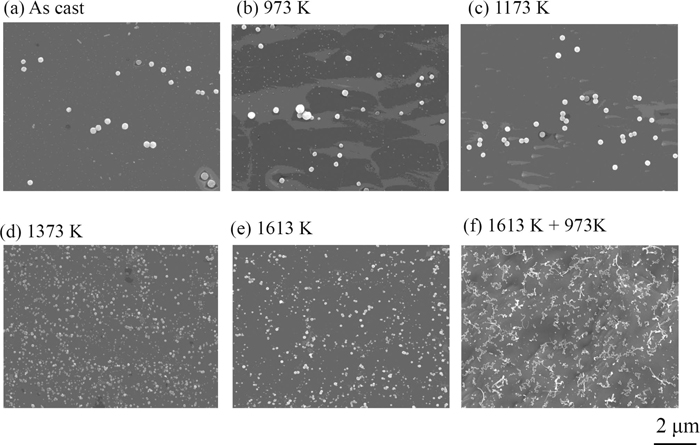
SEM images [(a)–(f)] of precipitates isothermally annealed at various temperatures.
Figure 4 shows TEM images [(a) and (b)] and EDS spectra [(c) and (d)] of precipitates in the heat-treated samples at 1613 K and at 1613 K followed by reheating at 973 K. From the EDS spectra [(c) and (d)], both the fine spherical precipitates are copper sulfides. All the precipitates we could find in both the samples were copper sulfides and other precipitates could not be confirmed, consistent with the XRD results as discussed below.

TEM images [(a) and (b)] and EDS spectra [(c) and (d)] of precipitates in the heat-treated samples at 1613 K and at 1613 K followed by reheating at 973 K, respectively. (e) An Electron diffraction pattern of the finely dispersed particle with zone axis [01-1].
Figure 5 shows XRD spectra taken from extracted precipitates in as-cast, isothermally heat-treated and two-stage heat-treated specimens. In the as-cast sample, the XRD peaks were identified to be reflections from the hexagonal FeS (JCPDS: 037-0477) and the cubic Cu2-xS (JCPDS: 024-0061), which is consistent with the results of the TEM examination shown in Fig. 2. The reflections due to the cubic Cu2-xS were clearly observed in the spectra of the as-cast sample and the heat-treated one at 973 K. In the spectrum of the sample heat-treated at 1173 K, the reflections due to the cubic Cu2-xS almost disappeared and only the reflections due to the FeS were clearly observed, which suggests that the cubic Cu2-xS is almost dissolved at 1173 K. In the spectra of the samples heat-treated at 1373 K and 1613 K, only the reflections due to the hexagonal Cu2-xS (JCPDS: 023-0958) were identified.

XRD spectra of precipitates isothermally annealed at various temperatures. Peak assignment was carried out based on the comparison with JCPDS cards. Cubic Cu2-xS (024-0061), hexagonal Cu2-xS (023-0958) and FeS (037-0477). (Online version in color.)
Assuming that dissolved Cu and S in the ferritic steel above 1173 K cause fine precipitations of the meta-stable hexagonal Cu2-xS during cooling,19) we can conclude that the cubic Cu2-xS precipitates almost dissolved at least 1173 K in the Fe-3.2%Si-0.4%Cu-0.02%S ferritic steel. Indeed, according to a Ref. 25), the hexagonal Cu2-xS has been proposed to be a meta-stable form of digenite (cubic Cu1.8S). In order to confirm this assumption, we show the XRD spectrum of precipitates in the heat-treated samples at 1613 K followed by reheating at 973 K. One can notice that the crystal structure of Cu2-xS is cubic in the sample, consistent with the electron diffraction patterns in Fig. 4(e). This implies that the hexagonal Cu2-xS is thermally meta-stable phase in the ferritic steel and the hexagonal Cu2-xS is transformed to be the cubic one.
Taking into account our experimental results as well as the reported suggestions,25) the hexagonal Cu2-xS seems to be extrinsic precipitates after heat treatments,19,26) that is, the hexagonal Cu2-xS does not exist in the thermal equilibrium state diagram but the cubic Cu2-xS only exist in the thermal equilibrium state diagram. From the experimental results, we consider that the cubic Cu2-xS almost dissolved at least 1173 K in the Fe-3.2%Si-0.4%Cu-0.02%S ferritic steel.
In order to confirm the precipitation temperature of the ferritic steel, the thermal stability of the cubic Cu2-xS is discussed based on the thermodynamic analysis. Figure 6 shows the solubility products of the cubic Cu2-xS reported in Refs. 16) and 21) comparing with our result obtained by the CALPHAD (Calculation of Phase Diagrams) method.27)
| (3) 27) |
| (4) 28) |
| (5) |
| (6) |
| (7) |

Precipitation behaviors of copper sulfides in the Fe-3.2%Si-0.4%Cu-0.02%S ferritic steel were investigated by XRD, TEM and SEM. Coarse spherical precipitates and rectangular precipitates were observed in a non-heat-treated sample. From the experimental results, we suggest that the rectangular precipitate is the cubic Cu2-xS and the coarse spherical precipitate is FeS. XRD reflections due to the cubic Cu2-xS were observed in a non-heat-treated sample and a specimen heat-treated at 973 K. The hexagonal Cu2-xS instead of the cubic Cu2-xS was identified by XRD in the samples heat-treated at 1373 K and 1613 K followed by quenching in iced water. According to the calculated solubility product based on the CALPHAD method, the dissolution temperature of the cubic Cu2-xS in the Fe-3.2%Si-0.4%Cu-0.02%S ferritic steel is predicted to be 1164 K. Taking the experimental results and subsequent thermodynamic calculations into account, we can conclude that the cubic Cu2-xS in the Fe-3.2%Si-0.4%Cu-0.02%S ferritic steel is almost dissolved at least 1173 K. This means that the solubility product of the cubic Cu2-xS is larger than those of MnS and FeS in the ferritic steel.
We thank NSST for the technical supports to our experiments. We thank T. Kubota, K. Kawakami and W. Yamada of NSSMC for invaluable advises for thermodynamic analysis in this work.