2016 Volume 56 Issue 3 Pages 436-443
2016 Volume 56 Issue 3 Pages 436-443
Electrochemical hydrogen permeation tests were carried out under controlled temperature and humidity using pure Fe sheet specimens outdoor-exposed at Beijing and Chongqing, China and Okinawa, Japan in order to understand the effect of environmental factors on hydrogen uptake behavior. The maximum hydrogen permeation current densities of the exposed specimens were in the order of Beijing > Chongqing >> Okinawa, while the order of the degree of corrosion of the specimens was Okinawa > Chongqing >> Beijing. X-ray fluorescence analysis showed that the surface concentrations of sulfur on the Beijing- and Chongqing-exposed specimens were higher than that of the Okinawa-exposed specimens, whereas chlorine concentrations of the Beijing and Okinawa-exposed specimens were higher than that of the Chongqing-exposed specimen. Nitrate concentrations of Beijing- and Chongqing-exposed specimens evaluated using nitrate test strips were obviously higher than that of the Okinawa-exposed specimens. It is suggested that air pollutants such as SO2 and NO2 and particulate matters containing inorganic acid ions, likely surfate and nitrate ions, and possibly organic acids contribute to acidification of rust layer leading to the enhanced hydrogen entry.
The evaluation of susceptibility of high strength steels to hydrogen embrittlement is of the great importance for safety and reliability of the usage and development of high strength steels. The critical hydrogen content for delayed fracture (hydrogen embrittlement) (HC) has been regarded as one of the determining factors for delayed fracture of high strength steels.1,2,3) HC and the relationship between fracture stress and diffusible hydrogen content have been examined by using constant load test,1,2,3,4) slow strain rate test (SSRT),5,6,7,8,9) conventional strain rate test (CSRT)10,11,12,13) of tensile specimens charged with hydrogen in conjunction with hydrogen content measurement by means of thermal desorption analysis (TDA).
As well as the importance of the effect of hydrogen content on the mechanical property, it is also required to understand the hydrogen entry behavior from the environment where the materials are used. Atmospheric corrosion is one of the causes of hydrogen entry and many of the high strength steels are used under atmospheric corrosion environment. Hydrogen entry caused by atmospheric corrosion is quite complicated because it is influenced by the characteristics of rusts, incorporated pollutants in rust and the changing environmental factors such as temperature and humidity.
Electrochemical hydrogen permeation test (ECHPT)14,15,16,17,18) is an useful technique to observe changes in hydrogen entry with time, and many researchers have taken advantages of ECHPT to elucidate hydrogen uptake behavior under atmospheric exposure and simulated atmospheric corrosion.19,20,21,22,23,24,25,26,27) In our previous study, it was found using ECHPT during cyclic corrosion test (CCT) that the rust later formed during atmospheric corrosion has an important role on enhancing hydrogen entry.25) ECHPT of Fe sheet specimen corroded by CCT24,27) and outdoor exposure27) under controlled temperature and humidity were also carried out for further understanding of hydrogen uptake behavior. The advantage of ECHPT under controlled temperature and humidity after outdoor exposure is that it is much easier than on-site ECHPT and that the exposure test can be carried out in any sites even in foreign countries where on-site ECHPT cannot be easily performed.
In order to understand the influence of environment on hydrogen entry behavior, Beijing and Chongqing, China and Okinawa, Japan were chosen as the sites for outdoor exposure tests in the present study, and ECHPTs of the exposed specimens were conducted under controlled temperature and humidity. Recently, it is often reported that the air pollution in metropolises in China is getting worse and one of our interests is the influence of such air pollution on hydrogen uptake. Okinawa where the coastal environment is corrosive due to relatively high air-borne salt deposition rate was also selected as an exposure site for comparison.
Commercially pure Fe sheets (The Nilaco Corporation, 99.5%) were used in this study. The specimen was initially 0.5 mm in thickness. The specimen was octagonal as shown in Fig. 1 and the effective area used for ECHPT was 20 cm2. After polishing of both sides of the sheet by using #800 grit SiC paper, one side was electroplated with Ni in Watt’s bath (NiCl2·6H2O 45 g/L, H3BO3 40 g/L, NiSO4·6H2O 250 g/L) at 60°C with a current density of 3 mA cm−2 for 180 s.22) The calculated thickness of Ni plating was about 180 nm.

Optical photographs of Fe specimens exposed at Beijing for 2 months (a) and 4 months (b); at Chongqing for 2 months (c) and 6 months (d); at Okinawa for 2 months (e) and 4 months (f).
The specimens were exposed outdoor with the Ni-plated side masked. The exposure sites were in Beijing (E 116.46, N 39.92), in Chongqing (E 106.54, N 29.59) and in Okinawa (E 127.50, N 26.20), and the exposure tests were started in January of 2013. After two and four months of exposure at Beijing, Okinawa and two and six months of exposure at Chongqing, the specimens were shipped back from the exposure sites to our laboratory to conduct ECHPT.
2.3. Hydrogen Permeation TestECHPTs of the exposed specimens were carried out in a constant temperature and humidity chamber. The experimental set up was the same as that shown elsewhere.24,27) The rusted specimen was fixed on a modified Devanathan-Stachurski cell, which was filled with a 1 M NaOH aqueous solution. A Hg/HgO reference electrode (E0 = + 99 mV vs SHE (25°C)) and a Pt counter electrode were set in the cell. The hydrogen output side was polarized at +0.1 V vs Hg/HgO and the measured hydrogen permeation current was recorded by using a data logger. The relative humidity was increased in stepwise manner (40, 50, 70, 80, 98%RH) at a constant temperature of 30°C. Each relative humidity from 40 to 80%RH was kept for about 2 h. When the set humidity of the chamber was 98%RH, a wet filter paper was used to cover the rusted specimen surface to keep specimen surface wet. The wet condition was kept for more than 10 h. After the wet condition, the wet paper was removed and the relative humidity was decreased in stepwise manner. The temperature and the relative humidity were monitored by using a thermometer and by a hygrometer, respectively, and they were recorded using the data logger.
2.4. Analysis of Exposed Specimen SurfaceTo analyze the corrosion products on the surface, pure Fe sheets with the size of 35 mm × 75 mm × 0.5 mmt were also exposed at each exposure site. After the exposure, the elemental composition of the corrosion products formed on the specimen surface were analyzed by means of X-ray fluorescence analysis (XRF) using a MESA-500W X-ray fluorescence analyzer, HORIBA, Ltd. The analysis area was 10 μm in diameter.
The concentration of nitrate on the rust surface for was roughly analyzed by using Quantofix® test strips for nitrate. About 0.02 mL of distilled water was put on the corroded surface by using a syringe. After 2 min the nitrate concentration in the solution droplet was estimated from the color change of the test strip.
Figure 1 shows the examples of optical photographs of the specimens exposed in Beijing and Chongqing, China and Okinawa, Japan. The specimen exposed at Beijing showed obviously less corrosion compared to the specimens exposed at the other sites, and most of the metallic surface was remained. Since the exposure test was started from January, the low temperature and low relative humidity of Beijing during the exposure test is presumably responsible for the less corrosion. Visual observation indicated that the degree of corrosion of the Okinawa-exposed specimen was more significant than that of the Chongqing-exposed specimen. The most significant corrosion of the specimens exposed at Okinawa is attributed to the subtropical and coastal environment of Okinawa.
Figure 2 shows SEM images of rust layer formed on the specimens exposed at Beijing, Chongqing and Okinawa for 2 months. Note that the SEM images were taken after ECHPT carried out in a constant temperature and humidity chamber and corrosion products newly formed during the test covered the surface. Initially the corrosion on the Beijing-exposed specimens was less obvious but application of high humidity resulted in significant formation of corrosion products on the surface probably because contaminants deposited during exposure test promoted corrosion. Significant difference was not seen visually with each other.
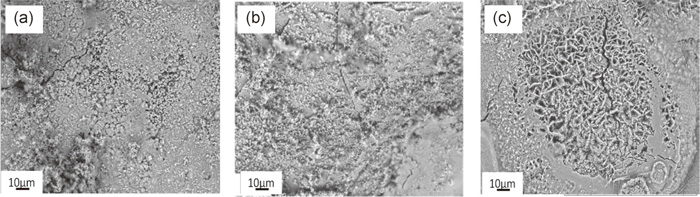
SEM photos of the surface of pure Fe specimens exposed at Beijing (a), at Chongqing (b) and Okinawa (c) for 2 months. The SEM photos were taken after electrochemical hydrogen permeation tests.
Figure 3 shows changes in the hydrogen permeation current densities of the specimens exposed at Beijing for 2 and 4 months with the change in applied relative humidity. The trends of the changes of hydrogen permeation current density were similar to each other, and the current density started to increase at 98%RH with application of wet paper on the surface. Before the wet condition was applied, the hydrogen permeation current density did not show obvious increase. The maximum hydrogen permeation current density of the specimens exposed at Beijing was about 0.15 μA cm−2. When the applied humidity was decreased after the wet condition, a small peak of hydrogen permeation current density was observed followed by relatively quick decrease.

Changes in hydrogen permeation current density with applied relative humidity for pure Fe specimens exposed at Beijing for 2 months (a) and 4 months (b).
Figure 4 shows the changes in hydrogen permeation current density for the specimen exposed at Chongqing for two and six months. The change of the hydrogen permeation current density of 2-months-exposed specimen was similar to that of the specimens exposed at Beijing, though the maximum current density of the specimen, about 0.1 μA cm−2, was smaller than that of the Beijing-exposed specimens. A slight increase of hydrogen permeation current density was recognizable when the humidity was increased to 70%RH.

Changes in hydrogen permeation current density with applied relative humidity for pure Fe specimens exposed at Chongqing for 2 months (a) and 6 months (b). The result of similar measurement repeated for the 6-month-exposed specimens is shown in (c).
A quite different trend of the change in hydrogen permeation current density was observed on the specimens exposed at Chongqing for 6 months. The hydrogen permeation current density was relatively low under wet condition but when the humidity was decreased the current density drastically rose, and then decreased. Further decrease in humidity to about 50%RH resulted in the appearance of the second current density peak. The same stepwise change of humidity was repeated for the specimen and the change of the hydrogen permeation current density was almost reproduced (Fig. 4(c)), although the corrosion product covering the specimen surface was increased during the previous ECHPT.
The hydrogen permeation current density of the specimens exposed at Okinawa showed no obvious increase when the humidity was increased, but when the humidity was decreased after applying wet condition a current peak appeared. The current peak looks similar to the first peak of the specimen exposed at Chongqing for 6 months that appeared during drying process. In our previous study,27) specimens exposed at Okinawa for 2 months showed slight increase in hydrogen permeation current under wet condition and a small peak appeared at the beginning of the drying process. Further decrease in applied humidity led to an appearance of a higher and broader current peak, which may correspond to the second peak of Chongqing-exposed specimens in the present study. The difference in the change of hydrogen entry behavior of Okinawa-exposed specimens for 2 months between the previous and the present studies is probably due to the difference in the characteristics of the rust layer dependent on the environment when the exposure test was carried out. The difference in the humidity-dependent hydrogen entry behavior of Chongqing-exposed specimens with exposure time is probably due to the change in the nature of rust layer with exposure time as well.
Similar ECHPT was carried out using a specimen exposed at Okinawa for 2 years. The exposure test was started in our previous study.27) Although the 2-month-exposed specimen in our previous study showed discernible increase of hydrogen permeation current density under wet condition and under the following drying process,27) the 2-year-exposed specimen showed no clear change in hydrogen permeation current density as shown in Fig. 6, except for a slight increase at the beginning of the drying process after the wet condition. The rust layer formed during the long time of exposure is probably resistant to corrosion more or less and it might result in the reduction of hydrogen entry. The decrease in hydrogen permeation current density implies that the formation of rust layer protective against corrosion probably suppress hydrogen uptake to some extent.

Changes in hydrogen permeation current density with applied relative humidity for pure Fe specimens exposed at Okinawa for 2 years.
Figure 7 shows the concentration of chlorine and sulfur on the rusted surface of specimens exposed at Beijing, Chongqing and Okinawa measured by means of X-ray fluorescence analysis. Chlorine detected on the specimens exposed at Beijing and Okinawa for 4 months was more obvious than that on the specimen exposed at Chongqing for 6 months. The data of Tsukuba, with rural environment, are also added in the figure for comparison. The origin of the chlorine on the specimens exposed Okinawa, with the coastal environment, is most probably air-borne salt from the sea. On the other hand, the origin of the chlorine on the Beijing-exposed specimen is barely due to air-borne salt because Beijing is geographically far from the coast so that chlorine might be derived from air pollution or snow melting agent. Winter in Chongqing is warmer than that in Beijing and the average lowest temperature in Chongqing in January, which is the lowest in the year, is 5.4°C. Therefore, Chongqing-exposed specimens might not receive chlorine originated from snow melting agent. The chlorine content of the specimens exposed at Tsukuba tended to be lower than that of the specimens exposed at Beijing and Okinawa.

Contents of Cl (a) and S (b) in the rusted surfaces of specimens exposed at Beijing, Chongqing and Okinawa. The concentrations were measured by means of X-ray fluorescence analysis. Cl and S contents of specimens exposed at Tsukuba for 2 and 4 months are added for comparison.
Significant difference in sulfur content was seen between the specimens exposed at Okinawa and Tsukuba, Japan and that exposed at Beijing and Chongqing, China. The level of sulfur contents of the specimens exposed at Okinawa and Tsukuba were almost the same and that were obviously lower than that of the specimens exposed in China. Most probably the higher sulfur content of the specimens exposed in China is due to air pollutant such as gaseous SO2, particulate sulfate or particulate matters containing such pollutants. SO2 changes to SO32− or SO42− ions by hydrolysis with the production of H+ (Eqs. (1) and (2))23) that accelerates corrosion and the acidification, and it presumably gives rise to enhancement of hydrogen entry.22)
| (1) |
| (2) |
Nitrate concentrations estimated by using test strips for nitrate are summarized in Table 1. Although the test method is qualitative, the result clearly revealed that the nitrate concentrations of the specimens exposed at Beijing and Chongqing were obviously higher than that of the specimens exposed at Okinawa. Nitrogen oxide reaction products, which are formed in the atmosphere, include both gaseous and particulate nitrates. The oxides of nitrogen, NOx (NO+NO2) mainly emitted in the atmosphere as NO, which is subsequently transformed into NO228) and it causes acidification as shown in the following equation.
| (3) |
| Beijing | Chongqing | Okinawa | |
|---|---|---|---|
| 2 month | 25 | 25 | <10 |
| 4 or 6 months | 50 | 50 | <10 |
| unit: mg/L | |||
Chloride, surfate and nitrate ions accelerate corrosion and they are considered to have influence on correspondent hydrogen entry. Acidification caused by sulfate and nitrate is considered to result in enhancement of efficiency of hydrogen entry. The specimens exposed at Okinawa with relatively high concentration of chlorine and low concentration of sulfur-related products and nitrate showed less hydrogen entry compared to the specimens exposed at the other sites in China although the degree of corrosion of the Okinawa-exposed specimens was most significant. Therefore, sulfate and nitrates deposited on the specimens in China seem to have dominant role on enhancing hydrogen entry. The coexistence of chlorine in addition to sulfur-related products and nitrate on the Beijing-exposed specimens is presumably the cause of the highest hydrogen entry of the specimen among the sites in this study. Thus, the substances deposited or incorporated in the corrosion product during atmospheric exposure have obvious influence on the hydrogen entry behavior.
In our previous study, similar ECHPT under controlled humidity was carried out for specimens after cyclic corrosion test (CCT) simulating atmospheric corrosion.27) One cycle of the CCT consisted of dry (50% RH, 5.75 h), wet (98% RH, 1.75 h) and salt spray (0.5% NaCl aqueous solution, 0.5 h) stages. The hydrogen permeation current density of a specimen exposed at Beijing for 2 months is compared with that of the specimens corroded by means of 30 and 60 cycles of CCT in Fig. 8.

Comparison of the changes in hydrogen permeation current densities of pure Fe specimens corroded by exposure at Beijing for 2 months and by CCT.
The Beijing-exposed specimen showed similar hydrogen permeation current density to the CCTed specimens. When the humidity was decreased to 50%RH after wetting the hydrogen permeation current of the Beijing-exposed specimen was decreased sharply whereas a current peak appeared for the CCTed specimens during drying process. The CCTed specimens were covered with thicker rust layer comparing to that on the atmospherically exposed specimen. Probably the longer time required for drying the thicker rust layer formed on the CCTed specimen is the reason for the slower decrease in the hydrogen permeation current density of the CCTed specimens.
It has been reported that the hydrogen permeation current density of the specimens corroded by using severely corrosive CCT increased with CCT cycles under wet condition and under the following drying process.27) Although the corrosion of the exposed specimen was obviously less than that of the CCTed specimens, the hydrogen permeation current density of the Beijing-exposed specimen under the wet condition was almost the same as the specimen experienced 60 cycles of CCT. This fact suggests that the substances deposited during outdoor exposure in Beijing are remarkably effective to enhance hydrogen entry.
In Fig. 9, the change in hydrogen permeation current density of a specimen exposed at Beijing for 2 months is compared with that measured under electrochemical hydrogen charging at −4.5 μA cm−2 in a 1 M NaOH solution.25) The cathodic hydrogen charging and increasing relative humidity were started at t=0 in the figure. The increase of hydrogen permeation current density under cathodic hydrogen charging is markedly faster than that of the rusted specimen under wet condition. This fact indicates that the gradual increase of the hydrogen permeation current density of the rusted specimen is not diffusion-controlled build up but it is caused by an increase in hydrogen uptake on the rusted specimen surface, in other words, the surface hydrogen concentration is increasing with time.

Comparison of the change in hydrogen permeation current densities of a pure Fe sheet specimen measured under cathodic hydrogen charging in a 1 M NaOH solution and that of a Fe sheet specimen exposed at Beijing for 2 month measured in a constant temperature and humidity chamber. For comparison, the hydrogen permeation current densities were normalized by the maximum current density. In the case of the Beijing-exposed specimen, the average hydrogen permeation current density was used as the maximum current density.
Since the surface condition is changing with the progress of corrosion especially under wet condition, it might lead to the gradual increase in hydrogen permeation current density. Similar change in relative humidity was repetitively applied to the specimen exposed at Beijing for 2 months. Similar change in hydrogen permeation current was observed as can be seen in Fig. 10, though the hydrogen permeation current density was decreased with the repetition. The decrease is probably because of the decrease in effective concentration of corrosive contaminants with the growth of rust layer and incorporation of the contaminants in the rust layer, presumably resulting in decline of corrosion rate and acidification.

The change in hydrogen permeation current density of a Fe sheet specimen exposed at Beijing for 2 month. Similar stepwise change of humidity was applied to the specimen repetitively. The number in the figure shows the order of the experiment carried out.

Change in potential of a specimen exposed at Chongqing for 6 months measured by means of Ag/AgCl reference electrode attached on the rusted surface of the specimen. The set relative humidity is also plotted in the figure.
The obvious increase in hydrogen permeation current density was seen only when wet condition was applied to the specimen exposed at Beijing for 2 month and for 4 month (Fig. 3(b)) and the specimens exposed at Okinawa (Fig. 5) as well, although corrosion takes place at lower applied humidity. This implies that hydrogen entry is not directly controlled by corrosion rate but other factors such as acidification caused by hydrolysis of ferrous ions and change in potential23,25,27) enhancing efficiency of hydrogen reduction reaction as cathodic reaction.

Changes in hydrogen permeation current density with applied relative humidity for pure Fe specimens exposed at Okinawa for 2 months (a) and 4 months (b).
Dissimilarly to the Beijing-exposed specimen and the specimen exposed at Chongqing for 2 months, the specimen exposed at Chongqing for 6 months showed two individual peaks during drying process after wetting. To look into the phenomenon, change in the potential with applied humidity was measured using Ag/AgCl reference electrode. The tip of the reference electrode filled with agar containing KCl was attached on the rusted specimen surface and the potential was monitored. Note that the relative humidity in the figure is not monitored humidity but set humidity.
With increase in the applied humidity the potential decreased. The sudden rising of potential took place when a piece of wet paper was put on the rusted surface. Under the wet condition, the potential was gradually shifted to the less noble direction. The decrease of the potential is correspondent to the gradual increase in hydrogen permeation current density under wet condition shown in Figs. 4(b) and 4(c). The increases in hydrogen permeation current density of the specimens exposed at Chongqing for 2 months (Fig. 4(a)) and at Beijing for 2 and 4 months (Figs. 3(a) and 3(b)) presumably had similar decrease in potential. The following drying process resulted in an increase in potential though the hydrogen permeation current density of the specimen exposed at Chongqing for 6 months showed two remarkable peaks during the drying process. Since the reference electrode is on the specimen surface, it is considered that the decrease in applied humidity did not directly affect the surface just beneath the tip of the reference electrode filled with agar and the measured potential. Nevertheless, the observed increase of potential at the beginning of drying process may imply that the appearance of hydrogen permeation current peaks does not distinctly correspondent to a decrease in potential.
So far, the influencing factors for the gradual enhancement of hydrogen entry under the wet condition and the two kinds of conspicuous enhancement of hydrogen entry during drying process indicated by the two hydrogen permeation current peaks are not understood well. Several factors are supposed to affect the change in hydrogen entry with the change in applied humidity, such as changes in potential, corrosion rate and pH22,23,29,30,31) related to change in the thickness of moisture film,32) chemical reaction of dissolved ions, concentration of anions causing corrosion and acidification such as chloride, sulfate and nitrate ions and change in the thickness of moisture layer or wetness of the rust layer during drying process. It is also supposed that the type of surface film, for instance formation of magnetite affects the hydrogen uptake.23) Further investigations are required to clarify the influencing factors of enhancement of hydrogen uptake. Since additional enhancement of hydrogen entry may be possible to occur under some circumstances, to survey conditions applied to the rusted specimens are important as well.
In the present study the effect of pollutants such as sulfur-rerated substances and nitrate detected on the surface of the specimens exposed at Beijing and Chongqing were higher than that on the specimens exposed at Okinawa and Tsukuba. Recently, air quality index (AQI) is open to public and we can check it on the net.33) For example, the range of AQI of SO2 on 28 Jan. 2014 is 4–61 in Beijing, 16–72 in Chongqing and 0–2 in Okinawa. The range of AQI of NO2 is 8–41 in Beijing, 21–45 in Chongqing and 2–6 in Okinawa. Apparently, air pollution in China is more than that in Japan. AQI of particulate matters such as PM10 and PM2.5 whose size are 10 μm and 2.5 μm or smaller, respectively, measured in China and Japan also show difference and the AQI value of PM2.5 in China often exceeds 150 or even higher. Since particulate matters contains SOx and NOx-related species, the higher AQI of the particulate matters should be related to the enhanced hydrogen uptake of the specimens exposed in China. In addition to the inorganic acids, water-soluble organic acids34,35) including di-carboxylic acid, ketocarboxylic acid, fatty acids and benzoic acid, etc. are incorporated in particulate matters. Presumably deposited particulate matters containing inorganic and such organic acids have effect on enhancing acidification of the metal surface leading to raise the efficiency of hydrogen entry. It is also interesting to carry out similar test using specimens exposed other sites and to elucidate region-specific hydrogen entry behavior.
The hydrogen entry behavior of atmospherically corroded specimens was investigated by using electrochemical hydrogen permeation test of pure Fe sheet specimens under controlled temperature and relative humidity after outdoor exposure at Okinawa, Beijing and Chongqing. The conclusions are as follows:
(1) Electrochemical hydrogen permeation test after exposure is useful to elucidate the effect of region-specific environment on hydrogen entry behavior.
(2) Hydrogen entry into the specimens exposed at Beijing and Chongqing was more obvious compared to that of the specimens exposed at Okinawa presumably because the air pollution in China results in enhancement of hydrogen entry.
(3) Air pollutants such as gaseous SOx and NOx and particulate matters containing sulfur- and nitrogen-related substances and organic acids accumulated on the exposed specimen surface have significant influence on hydrogen uptake. Coexistence of chloride ion with the pollutants probably leads to further enhancement of hydrogen uptake as indicated by the Beijing-exposed specimens.
(4) The response of hydrogen permeation current density of exposed specimens to the change in applied humidity is strongly dependent on the exposure site and exposure time.
Thanks are due to Dr. Hastuty Sri of National Institute for Materials Science for help of potential measurements of rusted specimens under controlled temperature and humidity.