2017 Volume 57 Issue 5 Pages 782-790
2017 Volume 57 Issue 5 Pages 782-790
A CALPHAD type thermodynamic modeling of the Fe–C–S system was carried out, in particular, in order to provide accurate description of activity-composition relationship of Fe–C–S liquid phase. The liquid may be used as a solvent of ferrous scrap recycling. All available and reliable experimental data including phase equilibria and activity of S in the liquid Fe–C–S were analyzed, and a self-consistent set of Gibbs energy equations for the stable phases were obtained. In addition to the Gibbs energy equations, interaction parameters between S and C in the liquid were additionally estimated as functions of temperature: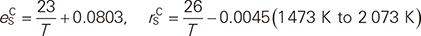
which were derived from the optimized Gibbs energy of the liquid phase using the Modified Quasichemical Model in the pair approximation. Moreover, the present thermodynamic modeling can be used to calculate various phase diagrams in the Fe–C–S system, and can be further extended in order to develop a multicomponent thermodynamic database.