2017 Volume 57 Issue 7 Pages 1166-1172
2017 Volume 57 Issue 7 Pages 1166-1172
For blast furnace iron-making, understanding the flow behavior of molten iron and slag on the surface of metallurgical coke will lead to optimal design of burden material, which strongly influences the gas-permeability of the furnace. Since the surface structure of coke changes as the reaction of carbon with CO2 or iron oxide progresses, wettability and motion of liquid in the coke-packed bed may be influenced depending on the reaction ratio of coke, i.e., the surface structure. In the present study, to investigate the influence of the surface structure of coke on liquid flow behavior, the advancing and receding contact angles of mercury and water on coke were measured. Of the two liquids, mercury has the higher surface tension. The surface structure of the coke substrate was varied by heating in CO–CO2 or CO2 atmospheres at 1273 K. It was found that the fine irregularities of the coke surface formed by the gasification reaction of the carbon increases the receding contact angle of water but has little influence on the dynamic contact angle of mercury. Depending on the results, influence of surface property of coke on flows of molten iron and slag in the blast furnace is discussed.
In the packed bed of a blast furnace, gas blown from tuyeres ascends, and molten iron and slag descend. Liquid holdup in the packed bed of coke decreases the gas permeability of the blast furnace and decreases the stability of the operation and productivity of the process. Liquid in the voids of the packed bed experiences a dragging force from the gas flow, and this may cause both of static and dynamic liquid holdup in the packed bed. It is known that excessive accumulation of liquid causes flooding and decreases the permeability significantly. Therefore, it is desired that liquids in the coke bed drop rapidly. The dripping behavior of liquid in a packed bed changes with variation of the liquid viscosity and wettability of the solid surfaces. Because the amount of liquid adhered to the packed material changes due to wettability, the amount of the static holdup of liquid depends on the wettability of the surfaces.1) In the case of wetting holdup, liquid is held in the vicinity of the contact point of packed material.2,3) On the other hand, in the case of non-wetting holdup, liquid accumulates on the site of concentrated, packed bed particles.4,5) In this way, the amount of liquid in the packed bed depends on surface tension, contact angle, and the structure of the packed bed. As represented by Young’s formula, the wettability is evaluated using the static contact angle, which is determined by the interface energy between the gas, liquid, and solid. With poor wettability, the contact angle has a value of 90° or more, and adhesion wetting by droplets can be observed. With good wettability, the angle is less than 90°, and immersional wetting is observed.
Measurements of the contact angle between the coke surface and liquid have been conducted.6) The contact angle between the carbon and slag of the CaO–SiO2–Al2O3–MgO system is in the range of 40°–160°, which is a wide range that depends on the concentration of FeO in the slag. The contact angle between the CaO–SiO2–Al2O3–MgO–FeO melt and carbon material varies from 125 to 105° at 1773 K with the resident time within 300 s.7) The interfacial energy decreases as the reduction reaction of FeO progresses. The contact angle between the CaO–SiO2–Al2O3–MgO–Fe2O3 melt and carbon material varies from 125 to 60° as the SiO2 is reduced;8) the wettability changes from poor to good as the reaction at the interface progresses. In summary, the contact angle between the carbonaceous material and the molten slag or iron will vary over a wide range depending on the composition of the liquid phase and the reaction at the interface.
To understand how to enhance the descent of liquid in the packed coke bed, one should consider liquid motion on the surface of a solid. Dynamic wettability changes with the resistance of movement of the triple line (i.e., the three-phase interface of solid-liquid-gas). In addition, advancing and receding contact angles have different values than the static contact angle. Moreover, the coke surface structure is a complex of uneven shapes and minute irregularities. The shape and concentration of ash on the surface change with the gasification reaction. Therefore, one must consider wettability on a non-smooth and irregular surface. In a previous paper,9) the contact angle of a moving droplet on a non-smooth solid surface was investigated, and the following results were obtained: (1) When the contact angle was approximately 90° or less, the drag force on the triple point increased as the surface roughness increased; (2) When the contact angle was significantly greater than 90°, the drag force on the triple line decreased. The effect of surface roughness changes with the degree of contact angle. Irregularity of the coke surface changes as the in-furnace reaction progresses, therefore the dynamic wettability of metallurgical coke used in actual furnace processes should be investigated.
In the present study, with the goal of better understanding the motion of molten slag and iron on the surface of coke in a blast furnace, we investigate the change in the dynamic contact angle of a liquid droplet on the coke surface. The coke substrate is reacted with CO/CO2 or CO2 gas at 1273 K, and the surface structure is changed. Advancing and receding contact angles of water and mercury droplet on the coke are measured at room temperature. Here, surface shape and reaction at interface affect wetting phenomena, however here in order to investigate only the influence of the surface shape, non-reactive systems were employed. Furthermore, the influence of the structure of the coke surface on the motion of the triple line is discussed.
To measure the advancing and receding contact angles at room temperature, ion-exchanged distilled water and reagent grade mercury were employed as specimens.
A coke substrate of 40×30×4 mm3 was prepared by cutting a sample of metallurgical coke of the kind used in blast furnaces. The surface was polished with #320 emery paper. Chemical properties of coke are shown in Table 1. The surface of the coke was treated by the following gasification reaction:
| (1) |
| mass% | |||
|---|---|---|---|
| Fixed carbon | Volatile | Ash | Water |
| 87.66 | 0.26 | 12.08 | 0.28 |
The coke substrate was held at 1273 K in a horizontal electric resistance furnace for 0–8 h. The atmosphere in the reaction tube was filled with a CO/CO2 ratio of 1/1 or with pure CO2 gas. Temperature, atmosphere and reaction time were determined assuming temperature and atmosphere of the thermal reserve zone, and the time for coke to move to below the cohesive zone, respectively. For the measurement of the contact angle of water, water repellent was finished to the coke substrate, it was first penetrated with a fluorine-based water repellent and then dried for 6 hours at 333 K. The contact angle of water on a solid surface using this fluorine-based water-repellent agent is about 100°. The surface roughness of the coke (Ra) was analyzed using a laser microscope.
2.2. Contact Angle MeasurementsThe advancing and receding contact angles were measured using an added-and-removed volume method. A schematic of the experimental apparatus is shown in Fig. 1. A substrate was placed horizontally, and a 2.5-ml syringe was fixed above it. Syringes without and with a hollow needle with an outer diameter of 0.50 mm were used in the experiments with, respectively, mercury and water. In order to measure the stable contact angles as the droplet advanced or receded, the expansion and contraction of the droplets were carried out slowly. In the experiments with mercury, to avoid the influence of hysteresis, 0.2 ml was injected onto the substrate over a period of 20 s, and then aspirated over a period of 20 s. In the experiments with water, 0.2 ml of water was injected onto the substrate over a period of 15 s, and then aspirated over a period of 15 s after the droplet stabilized. The shapes of the droplet during its expansion and contraction were captured in a high-resolution video using a camera mounted in a horizontal position. In order to clarify the outline of the droplet, LED lights were placed behind the droplet.
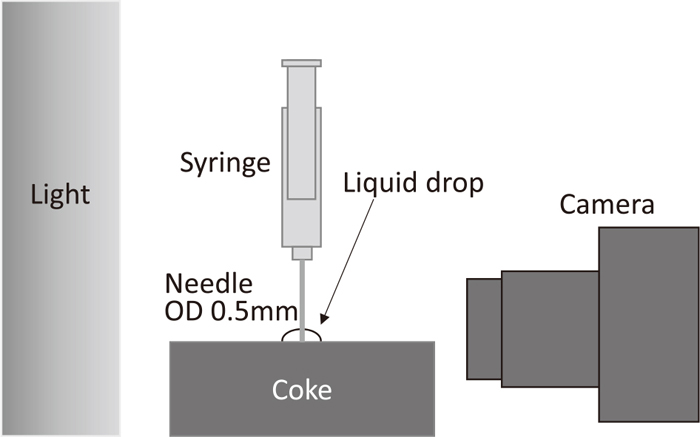
Schematic of experimental apparatus.
The method used to analyze the contact angle is the same as in the literature.9) Images of the water or mercury droplet during expansion and contraction were obtained from the movie. The contact angle was measured from the image. The position of the triple line and two positions on the contour of the droplet about few mm away from the triple line were read, and a circle passing through these points was drawn. Then, the angle between the substrate and the tangent to this circle was determined. The contact angle reading error using this method was less than 4°. The mean and standard deviation of the contact angles of contraction and expansion were calculated, and results are discussed in sections 3.C and 3.D.
Photographs of the coke substrates used for the experiments are shown in Fig. 2. Shown in the figure are samples (a) with no heat treatment, (b) heated at 1273 K in CO/CO2 for 2 h, (c) heated in CO/CO2 for 4 h, (d) heated in CO/CO2 for 8 h, and (e) heated in CO2 for 8 h. The weight of substrate decreased with an increasing in reaction time, the coke samples shrink slightly, and their color becomes darker.
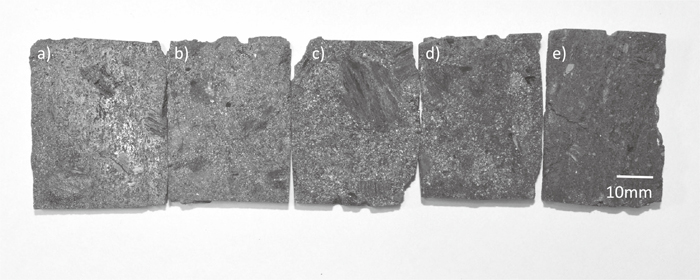
Coke substrates after heat treatment.
The weight and surface roughness (Ra) were measured for each substrate and are shown in Fig. 3, where the weight is expressed as a fraction of the initial weight. Using a laser microscope, the shape in the range of 4 mm in length of the sample surface was measured and Ra was obtained. Circles and squares represent, respectively, the weight ratio and Ra. Substrates treated in CO/CO2 and CO2 atmospheres are represented by solid and open symbols, respectively. The weights of samples heated under a CO/CO2 atmosphere decrease linearly with heating time. The weight reduction of the sample heated in CO2 is larger than that in CO/CO2. The influence of the heat treatment time and atmosphere on Ra is small. No relationship is observed between Ra and the weight change ratio. According to the observation of the coke surface, there are large pores more than 0.1 mm in size and fine irregularities formed on the coke matrix.
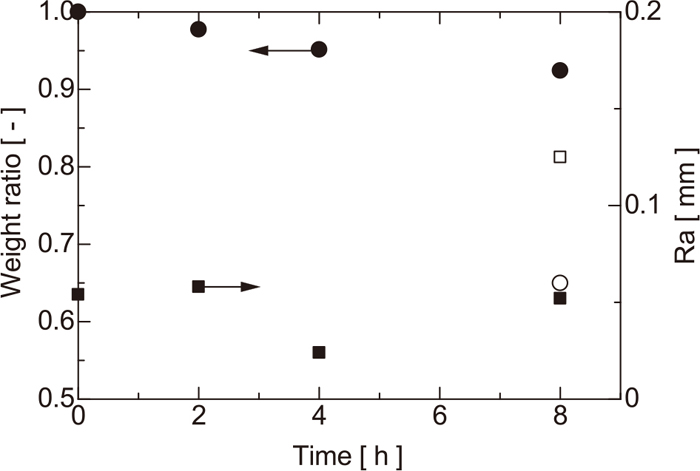
Weight ratio and Ra of coke with various treatment.
Metallurgical coke is made by coking of the coal. There are melted and un-melted parts, and, thus, the surface of the coke is irregular in structure and distribution of ash. Since only large and deep pores affect the present evaluation of roughness by Ra, fine irregularities are not sufficiently evaluated. It is difficult to carefully describe the surface properties of the cokes by using the Ra value alone.
Elemental distribution on the coke surface was analyzed using field-emission electron-probe microanalysis (FE-EPMA), and results are shown in Figs. 4(a), 4(b), 4(c) and 4(d) for, respectively, non-heat treated samples, samples heated in CO/CO2 for 4 h, samples heated in CO/CO2 for 8 h, and samples heated in CO2 for 8 h. White particles in the figure are ash, composed mainly of oxides. The shapes of the larger pores are not significantly changed by the gasification reaction of the coke; however, fine irregular structures were formed after heating, as shown in Figs. 4(b), 4(c), and 4(d). In addition, exposure of the ash on the coke surface increased with the reaction time.
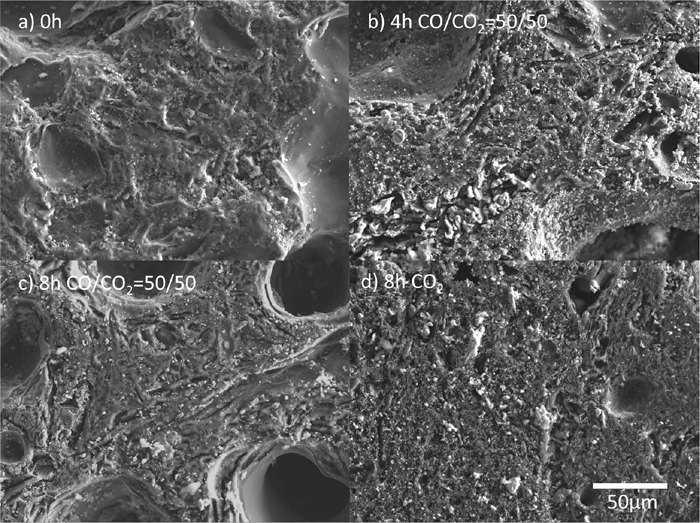
Surface of coke after heat treatment.
Distribution of oxygen and carbon on the surface (obtained by EPMA analysis) is shown in Figs. 5 and 6. The higher oxygen concentration region represents the presence of ash species. The area fraction of the ash region increases with the decreasing weight ratio shown in Fig. 3. This indicates that the concentration of ash on the surface of the coke increases with the gasification reaction with CO2 in the atmosphere.
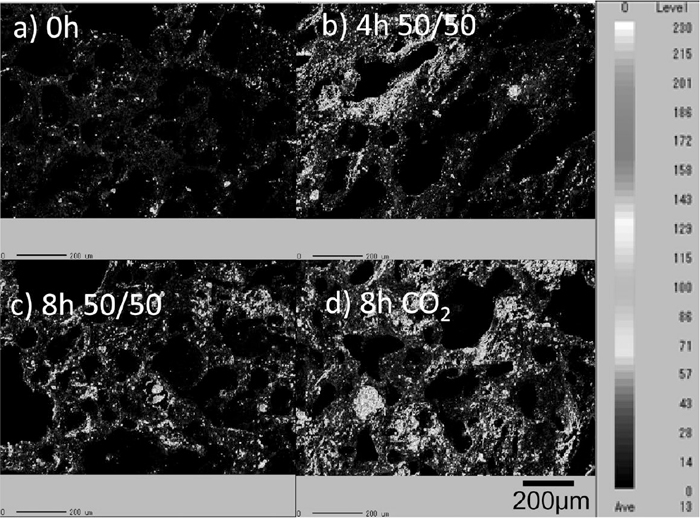
Relative concentration of oxygen on surface of coke substrate.
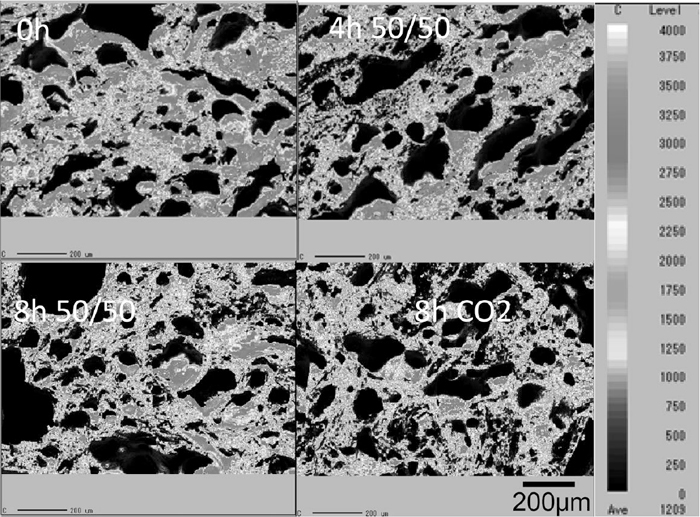
Relative concentration of carbon on surface of coke substrate.
Dynamic contact angles of mercury and water on coke were measured. Typical examples of droplet-shape are shown in Fig. 7, where panels (a) and (b) show advancing and receding contact angles of mercury, respectively, and (c) and (d) represent those of water. These liquids are injected and aspirated on the substrates. The trapezoid shape and vertical line are the tip of syringe and needle, respectively. The dark colored parts in the bottom of the photographs are the substrates. Therefore, the dynamic contact angle varied according to the time that the image was recorded. In a small droplet, the line energy at the triple line becomes large relative to the interfacial energy,9) and the contact angle may be affected by the energy of the coexisting gas–liquid–solid triple line. Thus, only images of droplets with diameters greater than 3 mm were used for the analysis.
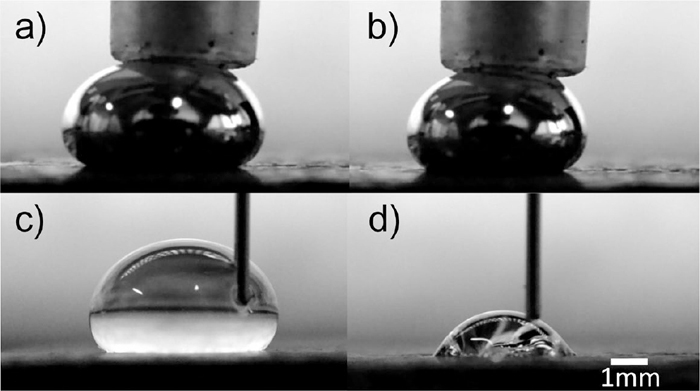
Shape of expanding and shrinking mercury and water droplet on coke substrate.
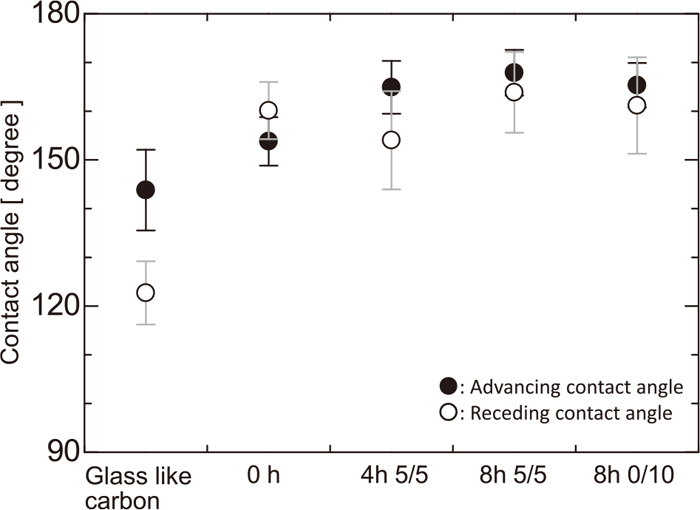
Advancing and receding contact angles of mercury on coke are shown in Fig. 8. Solid and open circles denote, respectively, the advancing and the receding contact angles. Labels on the horizontal axis represent the kind of the substrate. During each expansion and contraction event, six still images of the mercury droplet were captured from the movie. The position of the triple line moved intermittently in small increments and remained stationary between movements. Then, the average and the standard deviation are derived. For comparison, contact angles on glassy carbon9) are also shown as representative of angles on a flat substrate without pores. Advancing and receding contact angles of mercury on the coke substrates were within the range154–168°. The advancing angle is nearly the same as the receding angle, being only slightly larger. The contact angle slightly increases with an increase in substrate reaction time. Influence on the contact angles of the finer irregularities formed by the gasification reaction of coke is smaller than the difference of surface roughness between coke and glass like carbon. On the other hand, the advancing and receding contact angles on glassy carbon are smaller than those on coke; the wettability of mercury decreases as the surface roughness increases.
Advancing and receding contact angles of mercury on coke.
The critical adhesion contact angle of the droplet can be derived from the advancing contact and receding contact angles using the following relationship proposed by Furmidge:10)
| (2) 10) |
| (3) |
| (4) |
Since the surface tension of mercury at room temperature is constant, cosθr−cosθa represents the strength of the force applied on the liquid from the substrate. The relationship between the extent of the gasification reaction and the force-related parameter cosθr−cosθa is shown in Fig. 9. The vertical axis represents F/γgl, which is influenced by the contact area; therefore, the force cannot be directly compared in a precise sense. Compared to the force from the smooth surface of the glassy carbon, the force received from the coke surface is small. This means that surface roughness decreases the force on the mercury droplet. The force from the coke is almost zero. It should be also noted that the fine irregularities formed by the gasification reaction have little influence on the motion of mercury droplet.
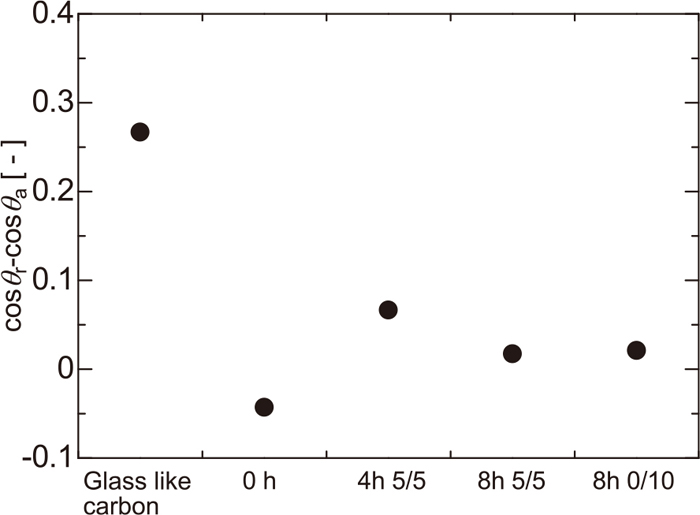
Difference between advancing and receding contact angle of mercury on coke.
Advancing and receding contact angles of water on the coke are shown in Fig. 10. Solid and open circles denote average advancing and receding contact angles, respectively. As in Fig. 8, labels on the horizontal axis represent the kind of the substrate. The error bar represents the standard deviation derived from six photographs. The advancing and receding contact angles on a flat substrate penetrated with water repellent were 131 and 68°, respectively.9) The advancing contact angle of water on coke varies within the range 152–169° and is larger than that on a flat substrate. Moreover, the advancing contact angle lies in the range 150–170°, and there is very little influence of heat treatment on the advancing contact angle.
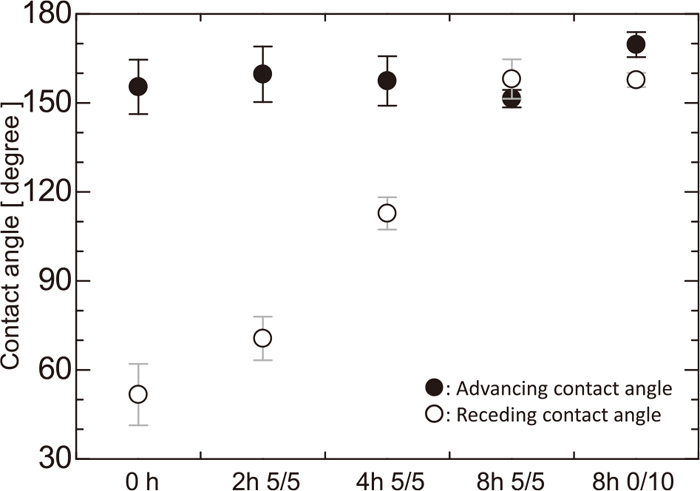
Advancing and receding contact angles of water on water-repellent coke.
The receding contact angle of water on coke varies over a wide range of 51–158° depending on the gasification reaction. The angle on un-heated coke is smaller than that on a flat substrate without pores; however, the angle on coke increases with the progress of the coke gasification reaction.
The values of cosθr−cosθa are plotted against the type of the substrates in Fig. 11. The value of cosθr−cosθa decreases with an increase in the gasification reaction. The value of (cosθr−cosθa/γ) for coke heated for 8 h is almost zero. A previous study found that the force required to move the droplet increases as Ra increases on a scratched substrate of uniform roughness.9) However, in this study, F changes independently of the roughness value represented by Ra meaning that the finer irregularities do affect the force value.
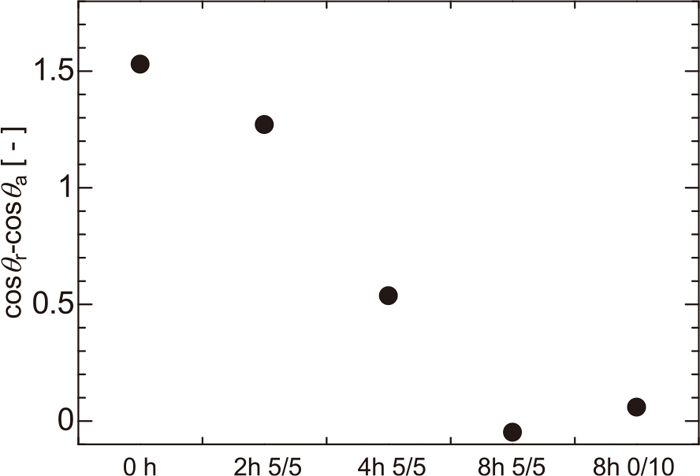
Difference between advancing and receding contact angle of water on water-repellent coke.
The advancing contact angles of both mercury and water on coke were larger than 150°; however, any influence due to the variation of the coke surface structure caused by the gasification reaction was not observed. Meanwhile, the variation of the receding contact angle does change depending on the surface condition. The receding angle of mercury was not changed by variation of the coke surface. On the other hand, that of water on coke without heat treatment was 52°, and it increases up to 160° as the heating time increases.
Variation of Ra of the coke surface measured by a laser microscope was not changed by the gasification reaction. However, FE-SEM observation clarifies that the fine structure of the surface changed, and the concentration of ash on the surface of the coke was increased. The contact angle of the droplet on coke was changed by the influence of the finer surface roughness formed by the gasification reaction, however the finer roughness was not detected as Ra. That of mercury was increased, while that of water was slightly changed for advancing contact and varied according to the surface condition for receding contact. Since Ra depends primarily on large pores, it cannot reflect the finer structural change caused by the gasification reaction. Change of the fine surface structure significantly affects the receding contact angle of water. In a previous paper, the influence of surface roughness on droplet motion on a water-repellent aluminum plate with a mirror finish or hairline scratches (Ra = 0.0026–0.028 mm) was investigated, but the variation of roughness had little effect on the receding contact angle of water. Consequently, roughness at the scale of several μm introduced by the gasification reaction increased the receding contact angle of water. The physical properties and contact angles with carbon of water,11) mercury,12) molten iron,13) and the CaO–SiO2–Al2O3 system14) are listed in Table 2.11,12,13,14,15,16) The contact angles given for water and mercury are for droplets on a water-repellent flat plate and glassy carbon, respectively. The surface tensions of mercury and water are, respectively, 465 and 72 mN/m, and the respective contact angles are 150 and 108°. In the condition of large contact angle or high surface tension, such as mercury on coke, fine irregularities on the coke surface have little influence on the contact angle. When the contact angle is close to 90° and the surface tension is small, fine irregularities on the coke surface have significant influence on the contact angle.
When there is a large difference in the magnitude of irregularities on the coke surface, one should consider not only the simple wettability at the interface of the solid and liquid, but also the complex wettability interface of the solid, liquid, and gas in the pores.17) Moreover, in the case of irregularities finer than in the present experiment, the relationship between the surface roughness and the contact angle has been reported to be non-monotonic.18) For the evaluation of the irregularity of the coke surface, including fine irregularities on a scale of several μm generated by the gasification of the surface, there is a need to clarify the relationship between size of the irregularities and the effect on the wettability.
4.2. Motion of Molten Slag and Iron on Coke SurfaceIn this study, dynamic contact angles of mercury and water were measured at room temperature. The influence of the coke surface after reaction with CO2 on the wettability was characterized. In an operating blast furnace, the same kind of gasification reaction of coke proceeds by reaction with CO2 or H2O.
Metallurgical coke used in a blast furnace is composed of carbon and ash and is irregularly shaped with pores of various sizes. Since the surface exhibits coarse irregularity, its wettability may be poorer than that on the flat cross sections used in this study. As obtained in present study on non-reactive systems, with consideration of the changes in the microstructure of the surface due to the gasification reaction, if the contact angle is slightly above 90° or surface tension is low, the receding contact angle will increase with the surface structural change, and then the resistance to droplet motion becomes smaller. For liquids with higher surface tension or contact angle higher than 150°, the liquid flow behavior may not depend on the gasification reaction of coke; these liquids would stably drop in the coke-packed bed with no dependence on the type of coke.
Variation of wettability of carbon with molten slag and iron has been previously summarized.6) The contact angle between the molten CaO–SiO2–Al2O3 system, which is representative of ironmaking slag, and graphite is around 160°. FeO or Fe2O3 in the slag decreases the contact angle of molten slag because of the reduction reaction, and the wettability may consequently become good. The contact angle between the molten Fe–C system and graphite increases with increasing carbon content, with values of 60° and 130° for 0% and 5% C, respectively. When the reduction of slag and carburizing of molten iron are in progress, the interface energy decreases because of these reactions, and, thus, the coke exhibits good wettability with the liquid phase just below the cohesive zone. The wettability then decreases with increasing reaction ratio because of the reduction of the slag. When the contact angle becomes greater than 90°, there is a possibility that the resistance to the liquid movement will be rapidly reduced.
In a previous study, we reported that the force on the liquid droplets from surface of a solid is reduced by a change from a wetting to a non-wetting state. Since the coke surface is changed by the gasification reaction in the blast furnace, we predict from this study that a more pronounced reduction of the force will take place. Therefore, an increase in contact angle, due to enhanced reaction between the coke and liquid-slag, would decrease the resistance on the descending liquid and, thus, increases the permeability of the dripping zone.
In this study, with the goal of understanding the motion of molten iron and slag on the surface of coke in a blast furnace, the dynamic contact angle of a droplet on a coke surface was measured. The coke substrate was reacted with CO–CO2 gas mixture or pure CO2 gas at 1273 K, and the surface structure was consequently changed. The relationship between the surface structure and the change in advancing and receding contact angles was investigated, and the following conclusions are obtained.
(1) The gasification reaction with CO2 increases exposed ash on the coke surface, and the finer irregularities (approximately several μm in size) are formed around the ash.
(2) Since the wettability is affected by the fine structure of the coke surface, it cannot be evaluated simply from the value of Ra, which is determined predominantly by the larger-scale pores.
(3) When the contact angle is close to or greater than 90°, the receding contact angle of liquid is increased because of fine irregularities formed by the gasification reaction on the coke surface.
(4) When the contact angle is greater than 150°, the influence of fine irregularities of coke surface formed by the gasification reaction becomes small.
Present research was carried out with the Environmental Researches Aided by Steel Foundation for Environmental Protection Technology. The authors express sincere thanks for the support.