2017 Volume 57 Issue 7 Pages 1197-1204
2017 Volume 57 Issue 7 Pages 1197-1204
Steelmaking slag is recycled and reused, but its utilization is restricted due to its chemical properties. On the other hand, steelmaking slag is considered to be a promising resource of iron and phosphorus in Japan. In this work, reduction of (FetO) and (P2O5) in steelmaking slag and recovery of iron and phosphorus resources were investigated with the aim of developing a new recycling process for steelmaking slag. In order to investigate the effect of slag basicity (%CaO/%SiO2) and FetO content in slag on phosphorus separation from steelmaking slag, steelmaking slags were reduced by carbonaceous material at high temperature by using an induction furnace. From the results, the effects of slag composition, temperature and oxygen partial pressure on phosphorus separation from steelmaking slag were discussed.
The ironmaking process generates approximately 290 kg/ton-steel1) of blast furnace slag, which is effectively utilized in construction materials and as a raw material for cement. The steelmaking process generates 120 kg/ton-steel1) of steelmaking slag, which is also utilized in construction materials. However, for use in roadbed material, it is necessary to reduce its hygroscopic expansion, which is caused by remaining CaO in the steelmaking slag. Some studies in which steelmaking slag is recycled to the ironmaking process to recover iron and CaO have been reported.2,3,4) For this purpose, however, phosphorus must be removed before recycling in order to avoid phosphorus contamination in the ironmaking process.
Some researches on phosphorus removal from steelmaking slag have been reported. For example, Shiomi et al.5) reduced synthesized steelmaking slag with basicity ((mass%CaO)/(mass%SiO2)) of 1.1–1.2 in a carbon crucible, and reported that the FetO in the slag was reduced before P2O5, and 68–94 mass% of the phosphorus in the slag was removed at 1773–1873 K. They also reported that the reduction rate of P2O5 in the slag by carbon was controlled by the chemical reaction. Takeuchi et al.6) investigated the influence of the coexistence of Fe–Si alloys on the reduction of steelmaking slag with basicity of 0.5–2.3 by carbon at 1873 K, and reported that phosphorus in the steelmaking slag was removed through the gas phase, and at least 60 mass% of the phosphorus was removed as a simple substance. Nagata7) conducted reduction experiments in which pretreatment slag with basicity of 2.7 was melted in a carbon crucible. He reported that 70 mass% of the phosphorus was removed through the gas phase at 1896–1938 K and discussed the possibility of further phosphorus removal by slag stirring to increase the reaction area.
Moreover, Morita et al.8) and Toishi and Itoh9) investigated the influence of microwave irradiation heating on the reduction of steelmaking slag. Kubo et al.10) found that the constituent phases of dephosphorization slag could be divided into two phases by a large gradient magnetic field; one was a phosphorus-enriched phase with a low iron content, and the other was a phase containing very little phosphorus. They also discussed the possibility of recovery as a phosphorus source.
Matsui et al.11,12) investigated the influence of temperature on the reduction behavior of FetO and P2O5 for several slags with basicity ranging from 1.2 to 4.0, and reported that more than 50 mass% of the phosphorus in the slag can be removed under the condition that FetO activity is less than 0.01.
As described above, the influence of the FetO content in slag on P2O5 reduction has not yet been clarified. In this work, the influence of the FetO content in slag on the reduction and separation behavior of iron and phosphorus was investigated.
A schematic diagram of the experimental apparatus used in the steelmaking slag reduction experiments is shown in Fig. 1. In the experiments, 100 g of the slag sample and graphite powder were charged in a MgO crucible and heated to the set temperature at the average rate of 15 K/min in a high frequency induction furnace. The temperature was measured by a thermocouple at the top of the slag sample. The slag compositions and the experimental conditions are shown in Tables 1 and 2, respectively. Silica sand (SiO2>95 mass%) was used to adjust the slag basicity. Graphite powder with more than 99 mass% C purity was used as a reductant, and its weight was set to 1.5 times the stoichiometric weight necessary to reduce all the FetO and P2O5 in the initial slag sample. The particle sizes of both the slag sample and the graphite powder were adjusted to be lower than 0.425×10−3 m. After holding for 30 min at the set temperature, mechanical stirring and heating were switched off and the sample was cooled in the air. In the experiments in which the slag melted, about 10 g of the slag was sampled at intervals of 0, 10, 20 and 30 min after reaching the set temperature. After magnetically separating the sample into slag and metal, their weights were measured and their chemical compositions were analyzed. Here, the collected samples were ground to a particle size smaller than 0.25×10−3 m before analysis.

Experimental apparatus. (Online version in color.)
| CaO | SiO2 | Al2O3 | MgO | MnO | P2O5 | T.Fe | FeO | Fe2O3 | M.Fe | (%CaO)/ (%SiO2) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mass% | |||||||||||
| Steelmaking slag | 41.0 | 13.8 | 5.6 | 6.1 | 2.1 | 1.6 | 18.7 | 11.4 | 10.8 | 2.3 | 3.0 |
| Ch No. | Initial FetO (mass%) | Target (%CaO)/ (%SiO2) | Temperature (K) | Used slag | Rotation speed (rpm) |
|---|---|---|---|---|---|
| 1-1 | 12.1 | 0.5 | 1523 | Steelmaking slag + silica sand | 60 |
| 1-2 | 12.1 | 0.5 | 1623 | ||
| 1-3 | 15.9 | 1.0 | 1523 | ||
| 1-4 | 15.9 | 1.0 | 1523 | ||
| 1-5 | 15.9 | 1.0 | 1573 | ||
| 1-6 | 15.9 | 2.0 | 1623 | ||
| 1-7 | 18.8 | 2.0 | 1523 | ||
| 1-8 | 18.8 | 2.0 | 1623 | ||
| 1-9 | 18.8 | 1.0 | 1623 | ||
| 2-1 | 2.4 | 1.0 | 1473 | ||
| 2-2 | 2.4 | 1.0 | 1573 | ||
| 2-3 | 2.0 | 1.0 | 1673 | ||
| 2-4 | 3.0 | 2.0 | 1473 | ||
| 2-5 | 3.0 | 2.0 | 1573 | ||
| 2-6 | 4.9 | 2.0 | 1673 |
The time changes in the FetO and P2O5 contents in the slag samples are shown in Figs. 2 and 3. The sections shown by solid lines are periods when the set temperature was maintained, and those shown by broken lines are periods which were difficult to evaluate because the rate of temperature increase/decrease varied in each experiment.

Change of (FetO) during reduction experiments. (Online version in color.)

Change of (P2O5) during reduction experiments. (Online version in color.)
In Experiments 1-1 to 1-9, in which the FetO content in the slag was higher, more than 80% of the FetO was reduced. However, in the experiments in which basicity was 2.0 and in which basicity was 1.0 under 1573 K, only 10–30% of the P2O5 was reduced.
In Experiments 2-1 to 2-6 with the lower FetO content slags, 80–90% of the P2O5 in the slag with basicity of 1.0 was reduced at more than 1573 K, but only 40–70% of the P2O5 in the slag with basicity of 2.0 was reduced at 1673 K.
3.2. Mass Balance before/after ExperimentsAfter the experiments, the samples were separated into metal, magnetic slag and non-magnetic slag, then the weights and chemical compositions of these respective substances were evaluated. Figure 4 shows images of the samples after the reduction experiments with slag having higher FetO contents and slag basicity of 0.5 to 2.0 at 1523 K and 1623 K. In the experiments with the 0.5 or 1.0 slag basicity, most of the metal cohered and no magnetic slag was obtained because the slag sample melted. In the experiment with 2.0 slag basicity, metal cohesion was limited and a large amount of magnetic slag was obtained because the slag sample did not melt.

Images of metal and slag after experiments. (Online version in color.)
The mass balances of iron and phosphorus in each of the experiments are shown in Figs. 5 and 6, respectively. The vertical axis is the ratio of the iron or phosphorus weight contained in the initial slag sample.

Mass balance of Fe in experiments. (Online version in color.)

Mass balance of P in experiments. (Online version in color.)
In Experiments 1-1 and 1-2, in which the slag with higher FetO contents and 0.5 basicity was reduced at 1523 K and 1623 K, about 80% of the FetO and 60% of the P2O5 were reduced.
In Experiments 1-3 to 1-5, in which the slag with higher FetO contents and 1.0 basicity was reduced at 1523 to 1573 K, about 90% of the FetO was reduced but only 10% of the P2O5 was reduced. On the other hand, in Experiment 1-6, in which the slag was reduced at 1673 K, about 60% of the P2O5 was reduced but separation of reduced iron and phosphorus was not complete because most of the reduced phosphorus was absorbed in the metal.
In Experiments 1-7 to 1-9, in which the slag with higher FetO contents and 2.0 basicity was reduced at 1523 to 1623 K, about 80% of the FetO was reduced but only 10–20% of the iron was separated as metal, and the rest remained in the slag as iron particles. Although about 30% of the P2O5 was reduced, approximately 20% of the reduced phosphorus was absorbed in the metal.
In Experiment 2-1, in which the slag with a lower FetO content and 1.0 basicity was reduced at 1473 K, only 20% of the FetO was reduced and all the reduced iron remained in the slag as iron particles. Regarding the phosphorus mass balance, it was not possible to distinguish the phosphorus as oxide and that captured in iron particles because the metal sample was not large enough for chemical analysis. In Experiments 2-2 and 2-3, in which the slag was reduced at 1573 K and 1673 K, 80–90% of the P2O5 was reduced. Here, undefined phosphorus, which was considered to be removed to the gas phase, was observed. The ratio of this undefined phosphorus was 10% at 1573 K and 50% at 1673 K.
In Experiments 2-4 and 2-5, in which the slag with lower FetO contents and 2.0 basicity was reduced at 1473 K and 1573 K, virtually none of the P2O5 was reduced. On the other hand, in Experiment 2-6, in which the slag was reduced at 1673 K, about 40% of the P2O5 was reduced.
As shown in Fig. 6, a maximum of 55% of undefined phosphorus was observed. From this, it can be thought that vaporizing dephosphorization occurred during the reduction experiments. Matsui et al.11,12) suggested the possibility that the reduced iron absorbed the vaporized phosphorus because phosphorus enrichment at the surface of iron particles which were not in contact with the slag was observed by EPMA analysis. When the reduced fine iron particles absorb the vaporized phosphorus, it is considered that the amount of phosphorus removed to the gas phase increases under the condition of a smaller amount of iron particles. This consideration is consistent with the experimental results in Fig. 6 showing that slags with lower FetO contents had higher undefined phosphorus ratios.
Thermodynamic calculations were performed by FactSage13) to estimate the form of the vaporized phosphorus. Figure 7 shows the relationship between temperature and the partial pressures of P2, P and PO for the slag shown in Table 2. Because P2 gas displays the highest partial pressure at the experimental temperatures in this study, the most stable form as a phosphorus-containing gas is P2. Therefore, the undefined phosphorus is considered as P2 in this report. The gas forms P3, P4, CP and the other phosphorus-containing gases are not considered here, as their partial pressures are smaller than one-thousandth of the partial pressures of P or PO.

Relationship between temperature and equilibrium partial pressure of P2, P and PO gases. (Online version in color.)
Figure 8 shows the relationship between the temperature and oxygen partial pressure necessary for the reduction of FetO and P2O5 in the slag to proceed. In Fig. 8, lines (1-1) and (1-2) correspond to Eq. (1), and lines (2)–(5) correspond to Eqs. (2), (3), (4), (5), respectively. Here, lines (1-1) and (1-2) express different conditions. Equations (6), (7), (8), (9), (10) shows the standard Gibbs energy14) for reactions (1)–(5).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |

Equilibrium oxygen partial pressures for various chemical reactions. (Online version in color.)
The assumptions used to calculate the relationship between temperature and the equilibrium oxygen partial pressure are shown in Table 3. The reaction for FetO reduction is shown by lines (1-1) and (1-2) assuming that Fe activity is 0.82 and FetO activity is 0.20 and 0.04, respectively. Here, Fe activity is calculated from the molar fraction of the iron which contains 4 mass% carbon and 1 mass% phosphorus assuming Raoult’s law. Line (3) shows the reaction in which reduced phosphorus from P2O5 is absorbed into the metal assuming that the activity of P2O5 is 0.01 and the metal composition is Fe-4mass%C-1mass%P. Line (4) shows the reaction in which CaO and P2O5 in slag generate Ca3P2, assuming that the activities of CaO, P2O5 and Ca3P2 are 0.3, 0.01 and 1, respectively. Line (5) shows the equilibrium reaction between carbon in the hot metal and CO gas assuming that the metal composition is Fe-4mass%C-1mass%P and the CO partial pressure is 1 atm.
Here, the activities of FetO, P2O5 and CaO were calculated by using the regular solution model proposed by Ban-ya15) for the slag compositions after Experiments 1-3 to 1-6 and 2-1 to 2-3. The metal compositions were assumed to be the same as those of the metal samples collected in the experiments, and the values adopted for the interaction parameters were
From Fig. 8, it can be stated that the reduction of FetO and P2O5 in the slag proceeds in the following order.
(a) Generation of metallic iron by reduction of FetO
(b) Absorption of phosphorus into the metallic iron after reduction of P2O5
(c) Progress of the reduction reactions in (a) and (b), and decrease in FetO content
(d) Evaporation of phosphorus into the gas phase after reduction of P2O5
This result is consistent with the experimental results shown in Chapter 3. In Experiments 1-1 to 1-9 with the higher FetO content slags, reduction of FetO and P2O5 starts at the oxygen partial pressure corresponding to line (1-1) in Fig. 8 as the above-mentioned (a) to (c). In Experiment 1-6 at high temperature, reaction (d) proceeds in addition to (a) to (c), and undefined phosphorus was observed. In Experiments 2-1 to 2-6 with the lower FetO content slags, reduction of FetO and P2O5 starts at the oxygen partial pressure corresponding to line (1-2) in Fig. 8 as reactions (a) and (d), and as a result, a large amount of undefined phosphorus was observed in Experiments 2-1 to 2-3.
The possibility of phosphide generation was also considered in Fig. 8. As shown in Fig. 8, the oxygen partial pressure determined by Eqs. (2) and (3) is 10−14 to 10−16 atm at 1473 K and 10−11 to 10−13 atm at 1673 K. For this range of oxygen partial pressures, the activity of Ca3P2 calculated from Eq. (4) is 3.05×10−9 to 10−17 at 1473 K and 3.67×10−9 to 10−17 at 1673 K. Thus, it can be stated that phosphide does not exist under the experimental conditions because the activity of phosphide is much less than the activity of P2O5.
4.3. Conditions for Phosphorus EvaporationPhosphorus evaporation is influenced by temperature, slag composition and oxygen partial pressure, but it is difficult to distinguish one influence from the others. Here, the phosphorus distribution ratio LP, which is a function of those three factors, is used as an index of phosphorus evaporation. The phosphorus distribution ratio LP is expressed by Eq. (11)
| (11) |
The phosphate capacity between gas and slag is defined by Eq. (12). Here, (X) and Y mean the component X in the slag and the component Y in the metal, and (%X) and [%Y] mean the concentrations (mass%) of component X in the slag and component Y in the metal, respectively.
| (12) |
By combining Eqs. (12) and (13), which express the P2 gas dissolution reaction14) into hot metal, Eq. (15) is obtained.
| (13) |
| (14) |
| (15) |
| (16) |
| (17) |
The activity coefficient fP is calculated by the carbon and phosphorus contents in iron droplets and the interaction parameters
Figure 9 shows the relationship between the calculated log LP and the undefined phosphorus ratio in each experiment. A good correlation exists between the two, and the undefined phosphorus ratio increases as log LP decreases. The results reported by Matsui et al.11,12) are also shown in Fig. 9. The undefined phosphorus ratio is less than 20% when slag with basicity of 1.0–2.0 is reduced at a temperature under 1673 K.
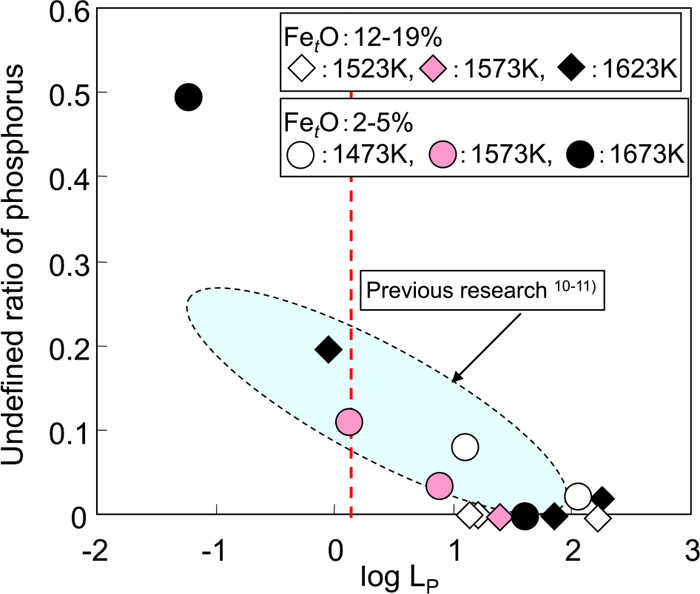
Relationship between log LP and undefined ratio of phosphorus. (Online version in color.)
The oxygen partial pressure used in Eq. (15) is the oxygen pressure equilibrated with FeO in the slag expressed in Eq. (1). By assuming the temperature and slag composition, the activity of FeO can be calculated from the regular solution model proposed by Ban-ya.15) This means that the oxygen partial pressure equilibrated with FeO in the slag can be expressed as a function of temperature and slag composition, and it can be stated that log LP can be expressed as a function of temperature, slag composition and metal composition from Eqs. (15), (16), (17). The relationship between temperature and slag basicity for log LP=0.1 is shown in Fig. 10 when the slag composition after reduction is (mass%Al2O3)=8, (%mass%MgO)=6, (mass%MnO)=6, (mass%P2O5)=2 and (mass%FetO)=0.5, 1.0 or 2.0. Here, the calculation was carried out under the condition log LP=0.1 because undefined phosphorus was observed under that condition in Fig. 9. From Fig. 10, the condition for phosphorus evaporation corresponding to the concentration of FetO can be obtained as the region below the lines for the respective FetO concentrations.

Relationship between temperature, slag basicity and FeO content in gaseous dephosphorization. (Online version in color.)
In order to clarify the effect of the FetO content in slag on iron and phosphorus separation from steelmaking slag, high temperature slag reduction experiments were conducted for slags with several FetO contents. The main results are as follows.
(1) The reduction ratio of FetO from slag with (%CaO)/(%SiO2)=1.0 and (%FetO)=15.9 mass% was more than 90% at 1523–1573 K. The reduction ratio of P2O5 from the same slag under the same condition was less than 10%. When slag with (%CaO)/(%SiO2)=1.0 and (%FetO)=2.0 was reduced at 1673 K, more than 50% of the P2O5 was undetected and is considered to be removed by phosphorus evaporation.
(2) The reduction ratio of FetO from slag with (%CaO)/(%SiO2)=2.0 and (%FetO)=18.8 mass% was more than 80% at 1523–1623 K. The reduction ratio of P2O5 from the same slag under the same condition was less than 30%. When the slag with (%CaO)/(%SiO2)=2.0 and (%FetO)=4.9 mass% was reduced at 1673 K, no P2O5 was removed from the slag
(3) The conditions for FetO and P2O5 reduction and phosphorus evaporation were discussed from a thermodynamic viewpoint. Under the condition that log LP is less than 0.1, which is calculated from the slag composition, oxygen partial pressure and temperature, some P2O5 was undefined. Under the condition that log LP is less than -1, the undefined ratio of P2O5 in slag was more than 50%.