2018 Volume 58 Issue 5 Pages 785-791
2018 Volume 58 Issue 5 Pages 785-791
It is well known that, the metallurgy has been honored as “the father of chemistry”. However, the metallurgy as an independent discipline was only established around the Second World War. Why does it take so long for this period? That is because for any matured science a quantitative description is required instead of a qualitative description. Most metallurgical systems are with large number of components and higher melting points that are difficult to be dealt with theoretically. As a result, the empirical treatment will be adopted instead of a theoretical model. There are two kinds of theoretical treatments: physical model and phenomenological model. The former one has a very clear physical picture but it is difficult to be treated. The later one is easy to deal with due to less number of variables. As a result, many metallurgical problems will be solved by using the later theoretical models. In this presentation, it will be shown that how we apply the phenomenological models to solve the metallurgical problems both in theoretical topics and technical aspects.
According to the history report, the earliest human productive activity was copper making and iron making that can be traced back to three thousand years ago. It was the earliest conscious production activity; therefore, one calls “metallurgy is the father of chemistry”. Nevertheless, the chemical metallurgy becomes a real science discipline that had experienced thousands of years until to the Second World War. Why does it take so much time? It was because the system to be treated in metallurgy is a multicomponent system and with high melting point that is very difficult to deal with both in theoretical treatments and experimental measurements.
A systematic treatment to the metallurgical systems was around the Second World War. The war required a large number of iron and steel which provided many practical data and at the same time it also raised a large number of questions to be investigated. As a result, a scientific branch, chemical metallurgy, has been gradually formed.
Professor Schenk from Achen University Germany first realized the importance of physical chemistry to metallurgy process who published a professional book, named, “Introduction to the Physical Chemistry of Steelmaking”1) that was the first text book of chemical metallurgy. Later, John Chipman from MIT started to design many kinds of high temperature experiments to extract various kinds of data from metallurgical systems.2) He made MIT become the center of metallurgical research. His research group members came from all over the world including China, Japan, Middle East, … and so forth. The Europe was also a center for chemical metallurgy, besides Schenk, Imperial College Richardson group, as well as Kubachewski … etc. have also made significant contribution to the chemical metallurgy. As to the theoretical work, Wagner and Darken … etc. have offered great contribution to this new field.
It is very important for chemical metallurgy to strengthen the theoretical research instead of empirical description. There are two kinds of theoretical models used to describe experiment data: physical model and phenomenological model. The first principle calculation belongs to the former one that is based on a real physical picture and serious mathematical treatment to derive various kinds of formulae. The second one is called as a phenomenological method that is based on couple of “axioms” or recognized facts, based on which a series of useful conclusions can be derived. The later one was mainly used by chemical metallurgy.
The development of chemical metallurgy in China can be summarized into two stages before and after 1949. In the first stage, Chinese scientists just like many our former colleagues joined into the foreign research group and engaged in many research topics in the chemical metallurgy. For instance, Wei Shoukun has been a post doctor studying in the Schenk’s group in 1935. It was probably the earliest one from China engaging in the chemical metallurgy research. Later, in 1937 Shao Xianghua went to England and studied in London University and Imperial College following Dr. H. Corpenter. Dr. Zou Yuan-xi (Chou Yuan-hsi) was another one working in the chemical metallurgy at the Carnegie Mellon University under Prof. G. Derge. After that he had built a wide connection with Chipman and Elliott at MIT. Dr. Chen Xinming had been Chipman’s PhD student directly under his guidance after the Second World War. Chen was famous in the measurement of Cr activity in iron. Of course there were even more Chinese scholars working in the chemical metallurgy at that time. The above four experts have continuously been working in this field with more influences in China. All of them have been elected as the Chinese Academician later.
The second stage from 1949 to 1980 is a special period time in China. We had lost the connection with the western foreign countries. However this kind of disconnection was “one way direction”. That means the Chinese scientists still knew the foreign colleagues’ activities including publications, however, the foreign scientists might not know what we were doing in China since we almost didn’t submit any our research work to western journals. Therefore it is necessary to let us introduce our situation to our foreign colleagues for that period of time.
The Chinese scientists educated from western country mentioned above still played a main role in chemical metallurgy researches in China. For instance, Prof. Wei Shoukun and other scientists had suggested and established a special class for “chemical metallurgy” with around 30 students each year. For this purpose he had even been in charge of a course for it and named “physical chemistry of metallurgical process”. It probably might be the first class for chemical metallurgy in the world.
Professor Wei’s lecture has been published as a text book named “Application of activity to physical chemistry of metallurgical process”3) in 1964, in which he has summarized the newest progresses in chemical metallurgy at that time. It was the newest summary for related papers of chemical metallurgy that included calculation of activity of metallurgical system, thermodynamic calculation for ternary and multicomponent systems, calculation of phase diagram, electric chemistry, … etc.. The contents of this book were so new that one could write paper just based on its content directly. Actually based on which Chou had written couple of papers.4)
After 1966 it was a difficult time for academic area in China, in that period of time all schools were closed, no paper, no research were allowed to be published. This period was lasting over ten years. After 1980 our country was opened to the western world again. We have started to publish our papers in western journals.
As discussed in the beginning of this presentation, the progress of chemical metallurgy was developing slowly due to the difficulty of “quantitative description” for many metallurgical processes. As a result, the empirical relations have been applied that will not be helpful for further investigation. Based on this situation, the phenomenological theory has been introduced to our problems and it is hoped that will be useful for solving our problems quantitatively. In this report, we will introduce five examples—three theoretical topics and two technique examples, to see how to use phenomenological theory to get solution done.
3.1. Calculation of Physicochemical Properties of Melts in Multicomponent SystemsPhysicochemical properties of solution for multicomponent systems are very important for many fields, such as, metallurgy, materials science, chemical engineering, medical, agriculture, environment … etc.. Nevertheless, this problem has not been solved yet for a long time. For binary systems there are a number of data, however, for ternary system and up the data are very limited. The experimental methods are very difficult to solve this problem. One has to go to solve this problem based on theoretical calculation due to its huge numbers of systems. There are two kinds of calculation methods “physical model” and “phenomenological model”. The former one has a very clear physical picture and a detailed theoretical deduction steps. Nevertheless, its practical application still has a long way because lack of many structure information and accurate results. In the practice, it still relied on empirical methods, for instance, especially on the method of calculating ternary properties in terms on binary ones. This method began in the early 20th century, Scatchard5,6,7) and his group from MIT had done a lot of researches in this topic for both symmetric systems and asymmetric ones. In 1960, a group of researchers8,9,10) from material sciences developed another model system for calculating properties of ternary system from binaries;11) we called this new model as “geometrical model” due to its relying on a geometrical plot.12) The author has proved that these two model systems actually are equivalent. This fact leads two important conclusions: (i) the geometrical model (or numerical model) has not made any further progresses after this half century; (ii) though the first principle method can give a very clear physical picture and parameter meaning, it is still not able to replace the empirical model in providing practical data. The above two facts lead to one conclusion, that is, “the empirical model cannot be replaced but needs to improve”. How to improve? This way directs to phenomenological model. Based on the above fact and conclusion, we have made our effort in improving geometric model. The direction points to make the theoretical method reasonable. As we pointed out in our series of papers, all current geometrical models are not reasonable due to its incorrect selection of “binary representative point”. Based on our analysis, a reasonable selection for “binary representative point” has been proposed that varies with the system itself.13,14,15) Our new model is described as follows.
The physicochemical properties in a multicomponent system ΔGE can be expressed as a combination of the corresponding properties
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |

The selected binary composition points for new model.
In sum, all foregoing models for calculating properties of multicomponent system based on the corresponding binary properties are incorrectly assumed that the “selected binary representative point” is fixed and independent of the system treated, that will cause the calculated property of a ternary system cannot reduce to a lower binary system even though when two components are identical. Our new model meets this theoretical requirement; therefore, it is a phenomenological model.13,14,15) This kind of model has already been paid attention by many readers due to its theoretical correction16,17,18,19) and used to higher component systems,20) up to now it has been cited over two hundred times. Some authors even did some comparison for different methods and based on which they had confirmed the correction of our new model.20,21,22,23,24,25,26,27,28,29) Besides we have also considered the calculation in a miscibility area.30,31,32,33)
The above phenomenological model deals with various kinds of properties for solution in a whole concentration range. For some metallurgical slags our group has also summarized some regulations for viscosity and conductivity that belong to semi-theoretical and semi-empirical models. They are also very meaningful for practical systems.34,35)
3.2. Calculation of Activity from Phase DiagramIn the previous section we have discussed how to calculate the properties of multicomponent systems based on binary ones. In this section we will further discuss how to get binary data. It is well known that, the phase diagram is a big data source for thermodynamic properties, from which one can find the activity, melting point, heat capacities, enthalpy, Gibbs free energy, partial entropy, … and so forth. It is also well known that, sometimes the activity of component is difficult to measure due to the higher melting temperature or caustic environment in experimental conditions. Therefore, the theoretical calculation of activity is meaningful.
Richardson had proposed a method for calculating activities based on the phase diagram that contains a series of intermediate compounds, and for which the Gibbs free energies of formations are known. Later Chou Yuan-hsi developed a new method based on only one intermediate compound. The above works are all based on the phase diagram with intermediate compounds. It is well known that there are many kinds of phase diagrams. The different phase diagrams should have different calculation methods. Our group has developed all kinds of methods for all possible binary phase diagrams including simple phase diagrams with complete insolubility in solid, phase diagrams with partial dissolubility in liquid or solid phase, … and so forth.
In this section we would like to give an example that shows how to calculate the activity from a phase diagram with an intermediate compound AηBξ (shown in Fig. 2) when the entropy of formation of this intermediate compound
| (6) |

Representative diagram with an intermediate compound.
Please note the method introduced here is based on the relations of thermodynamic function that is different from a regression method. In our method all functions have their own physical meanings and their corresponding values are all fixed and won’t be changed with the number of data points.
3.3. Kinetics of Metallurgical ReactionThere are a series of consecutive reactions from ore to metal in a metallurgical process. The reaction rate is concerned since it is directly related to productivity. From point of view of reactant phases, there are totally six kinds of reactions: solid-gas, solid-liquid, solid-solid, liquid-gas, liquid-liquid and gas-gas reactions, in which the most important type of reaction is the gas-solid reaction. It is because, not only the gas-solid reaction plays a very important role in the practical metallurgical processes but also the way of its theoretical treatment can also be referenced by rest kinds of reactions. It is well known that the reactions in blast furnace, converter furnace, flash furnace… all belong to a gas-solid reaction. Besides, the theoretical treatment for gas-solid reaction can also be referenced by gas-liquid, liquid-liquid, solid-liquid reactions in some manner.
Except for the reaction temperature, external pressure, particle size as well as particle shape, the main factor affecting the reaction rate is the reaction mechanism that is also a very complicated factor including many intermediate steps and each step will affect the total rate.45,46,47) Unfortunately, the foregoing treatments had been greatly simplified by so called Jander model,48) parabolic model49)… etc. that cannot meet the requirement of today’s metallurgy industry development.
In the past years, our group has made an effort in the metallurgical kinetic researches based on some theoretical considerations. We had considered the influence of various kinds of factors on the reaction rates: such as external temperature and pressure, the shape and size of particles … etc.. We had discussed these effects of varying temperature and pressure on the reaction rate, for instant46,50,51)
| (7) |
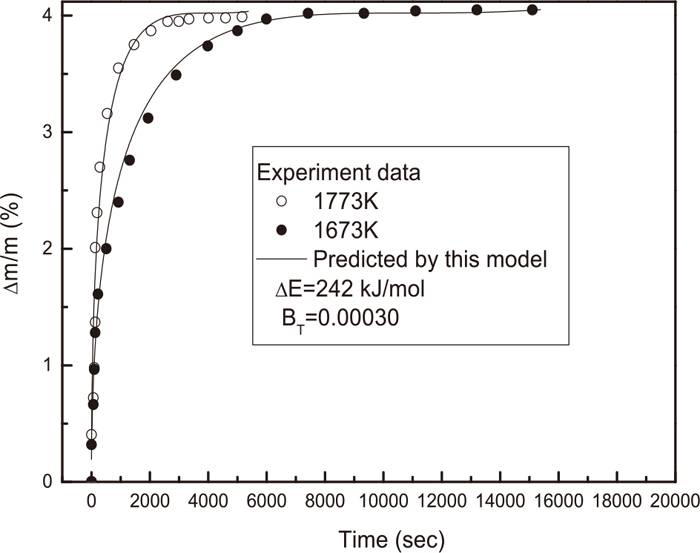
Isothermal oxidation curves of AlON powder at 1673 K and 1773 K.
The influence of different particle distribution on reaction rate had also been considered in our lab. Here is the formula52,53)
| (8) |
| (9) |
From the above Figs. 4 and 5, it is clear to show that, after considering the size distribution factor, the calculated results have obtained a great improvement.52,53)

A comparison of experimental data with regular model for oxidation of β-SiAlON powder at temperature 1373, 1473, 1573 and 1673 K, respectively.

A comparison of experimental data with model for oxidation of β-SiAlON powder at temperature 1373, 1473, 1573 and 1673 K, respectively.
The effect of oxidation product outside the particle on the oxidation rate has also been considered recently. A theoretical formula has been presented for particle shape of spherical one as follows54)
| (10) |
In addition to the factors mentioned above that have been considered in our group, we have also studied other more situations, such as temperature and pressure as a variable in the kinetic treatment … etc.. Owing to the limitation of the length of paper, it is omitted here. The interested readers might refer to our relevant papers.46,50,51,55)
Our researches have successfully been applied to many practical systems, such as hydrogen storage materials, ceramic system, metal and alloys oxidation as well as reduction of metallurgical ores. They all get good results. Besides, we have also considered the applications in metallurgical processes.56,57,58,59)
3.4. A New Technique: Deoxidation BallIn the above section, we have introduced how the theoretical method can be applied to the chemical metallurgy and further to establish some new theories. In this section, we are going to discuss how to use the theory to design some new technology directly.
The deoxidation is one of the important steps in the steel-making process. The key issue is that, the deoxidation inclusion is difficult to be removed out of the liquid steel due to the extremely small size. In order to solve this problem, we have proposed couple of electrochemical methods60,61,62) that have been published in some US patents63,64) and Chinese patents.65) At present we like to introduce our interesting patent named “deoxidation ball”66) as shown in Fig. 6, in which the ball shell is made by ZrO2 (or a kind of solid electrolyte). The metal aluminum powder is filled up inside the ball. Finally, the hole is sealed with a kind of cement that must be a kind of electronic conductor. When this kind of deoxidation balls are poured into the liquid steel, the oxygen inside the steel will be removed immediately and without any inclusion formed within liquid steel. This idea was come from our former theoretical analyses of short circuit deoxidation patent. The whole electrochemical reaction can be shown in the following steps:
| (i) |
| (ii) |
| (iii) |
| (iv) |
| (v) |
| (11) |
| (12) |

The structure of deoxidation ball.

The schematic mechanism of deoxidation ball.
It is easy to prove that the equivalent circuit of deoxidation ball is the same as the above short circuit processes. Therefore the Eq. (12) can be used for deoxidation ball either. This designed deoxidaton ball greatly simplifies the whole electric circuit system, and then greatly increases the electric conductivity, therefore, they should have a high deoxidation rate and more important is that, it completely prevents the deoxidation inclusion from entering into the liquid steel.
3.5. Measurement of Melting Temperature of Glass MaterialsIn the metallurgical process, one might use various kinds of slags for extracting and separating metals or alloys, for this purpose the melting temperatures are concerned. It is well known that there are a lot of cases that the slag is in glass states, in which the freedom degree of Gibbs phase rule is not equal to zero, in this case how to measure the melting point (or melting range) is a question. In general, the slag melting point can be measured by using the so-called cooling curve or heating curve technique. However, when the measured system is with a glass state or with a very small melting heat, that will cause the problem by using this kind of technique. The above situation can be explained in terms of the Fig. 8(a), it is a typical heating curve diagram that can be used as an experimental method to decide the melting point of a pure compound, in which, the curve is with a “horizontal segment” that means a process of melting is set in motion. if this heat effect is so small that one cannot find this “turning point”, under this situation a smooth curve with a small change in curve tangent should be expected (Fig. 8(b)). Under this situation one will be difficult to find the “glass softening point”.13)

A typical heating curve with different size sample. (a) Normal size crystal materials; (b) Normal size glass materials; (c) Fine powder glass materials.
This problem can be solved in this way, according to our derived formula for heating curve in the melting process of glass, that is
| (13) |
| (14) |
If some glass materials are difficulty to find the softening point due to smaller heat effect, let us grind the solid molten slag or glass materials into small powder, a turning point will appears around the softening point.
From above applications, it can be seen that, our researches have involved in the thermodynamics, solution model, phase diagram calculation, metallurgical kinetics, electro-chemistry as well as surface effect that are almost all contents concerned by physical chemistry. Therefore, it is expected that the applications of the phenomenological theory to physical chemistry of chemical metallurgy should have a very bright future.
(1) Chemical metallurgy is with its long history and the pioneer activity in the human production, it has been honored as “the father of chemistry”.
(2) However, the chemical metallurgy became a scientific independent discipline that takes thousands years until the Second World War. It is because a matured discipline needs theoretical quantitative work that is not easy for the chemical metallurgy due to the difficult treatment for the multicomponent systems with a high melting point.
(3) Around the Second World War, a group of scientific researchers who came from the United States and Europe started to establish the chemical metallurgical researches that could be regarded as the birth of chemical metallurgy.
(4) There are two kinds of theory for quantitative treatment: one is the first principle methods, another is the so called “phenomenological theory”. Though the first one has made great progresses recently, it is still a long way to get the practical applications in chemical metallurgical systems. The later one is still the major method for dealing with chemical metallurgical problems. However, it is expected that the combination of two kinds of theoretical methods will greatly promote the development of chemical metallurgy.
(5) The metal mineral resources is a kind of non-renewable resources, with the time going the rich ore becomes less and less, impurity is going to be more and more, re-use of secondary resources will be paid more and more attention. As a result, the metallurgist mission will be difficult but glorious.
The author would like to acknowledge financial support from the National Natural Science Foundation of China (Nos. 51734002, 51474141, 51174022, 50974084, 50774004, and 58670375).