2018 Volume 58 Issue 9 Pages 1629-1634
2018 Volume 58 Issue 9 Pages 1629-1634
In the process of hot-dip galvanizing of steel, alloying elements such as Si and Mn are easily oxidized by H2O in the annealing atmosphere, causing coating defects. Because this selective oxidation depends on the annealing heat pattern, i.e., the soaking temperature and time, basic research on the kinetics of selective oxidation is important for clarifying the phenomenon of selective oxidation. In this study, the effects of the annealing temperature and dew point on the kinetics and compounds of Mn external oxidation were investigated experimentally, and the Mn external oxidation rate was estimated based on a diffusion equation and thermodynamic equilibrium, considering the diffusion coefficient and the activity coefficient of Mn in steel. The amount of Mn oxide increased in proportion to the square root of the soaking time. This result suggests that Mn oxidation is a diffusion limited process. The Mn oxidation rate increased with increasing temperature and reached a peak value, and at higher temperatures, the Mn oxidation rate became dramatically slower. The peak value also depended on the dew point. To clarify the reason for this slowdown of Mn oxidation, the Mn oxidation rate was estimated. Considering the activity coefficient and the diffusion coefficient of Mn in steel, the calculated Mn oxidation rate was consistent with the measured value. It is thought that the Mn oxidation rate slows at high temperature because the gradient of the Mn concentration around the steel surface becomes small at high temperatures near the equilibrium temperature of Mn/MnO.
In recent years, concern about reducing automotive body weight and improving crashworthiness has increased dramatically, encouraging active use of high-tensile steel sheets containing additive elements such as Mn and Si.1,2) To achieve excellent anti-corrosion properties, these high-tensile steel sheets are used in galvanized components.3)
In the manufacturing process of galvanized steel sheets, cold-rolled steel sheets are annealed in an atmosphere-controlled continuous furnace in order to reduce surface oxides of Fe and to recrystallize the steel sheet structures. The steel sheets are subsequently galvanized by immersion in molten zinc. The atmosphere of the continuous furnace usually contains N2 gas with a H2 content of around 10 vol% and a dew point of less than −20°C. The annealing temperature ranges from around 700°C to 900°C. Because Fe oxide can be reduced but both Si and Mn can be oxidized thermodynamically in this annealing atmosphere,4) Si and Mn are oxidized on the sheet surface. It is well known that these oxides of Si and Mn deteriorate the wettability of molten zinc on the surface of steel.5,6,7,8) It is also recognized that this selective surface oxidation behavior depends on the conditions of the annealing heat pattern such as the heating rate and the soaking temperature and time, and that the compounds and amount of the oxides are affected by the dew point.9,10,11,12,13,14) Therefore, basic research on the kinetics of selective oxidation of Si- and Mn-bearing steel is important from the viewpoint of active utilization of galvanized high-tensile steel sheets. Some researchers have measured and calculated the kinetics of selective oxidation,15,16,17) but reports concerning the Mn oxidation rate under high temperature and low dew point conditions are rare. In previous study,18) the effects of the annealing temperature and dew point on the kinetics of Mn external oxidation were investigated experimentally. In this study, the compounds formed by Mn oxidation were analyzed and the Mn external oxidation rate was estimated based on a diffusion equation and thermodynamic equilibrium, considering the diffusion coefficient and the activity coefficient of Mn in steel.
The steel samples used in this study were full hard cold-rolled Mn-bearing steels with the thickness of 1.0 mm. The steel composition was 0.071 mass%C and 2.0 mass%Mn, as shown Table 1. The samples were precleaned by electrolytic degreasing, which was performed in an alkaline solution of 3.4 mass% NaOH at 500 A·m–2 for 10 s.
| C | Si | Mn | Al |
|---|---|---|---|
| 0.071 | 0.03 | 2.0 | 0.022 |
The samples were then pickled in an acidic solution of 5 mass% HCl at 60°C for 6 s, and annealed by heating in an infrared furnace to a temperature ranging from 700°C to 900°C and holding for 0 s to 300 s in an atmosphere of N2 + 10vol%H2 at a dew point of −35°C or −45°C. The heating rate was 10°C·s−1. After annealing, the samples were rapidly cooled to room temperature with 100 vol% N2 at a flow rate of 200 L·min−1.
2.2. Surface AnalysisSelective surface oxidation behavior was investigated by glow discharge optical emission spectroscopy (GD-OES). The conditions used in these measurements were a high frequency output of 35 W and an Ar gas pressure of 600 Pa. The sputtering time was 30 s, and the sputtering rate was approximately 0.06 μm·s–1.
The amount of selective surface oxidation was determined by wet chemical analysis. The surface oxides were dissolved with inhibited hydrochloride acid (HCl), and the produced solution was subsequently analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES). The Mn oxidation amount per unit area of the steel surface was calculated from the results of the ICP-OES analysis.
The surfaces of the annealed samples were observed by scanning electron microscopy (SEM). The conditions used in this observation were an acceleration voltage of 5 kV and working distance of 10 mm. Fourier transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) were used to characterize the selective surface oxides on the annealed samples.
Figure 1 shows the GDS depth profiles of the samples annealed at soaking temperatures of 700°C, 800°C and 900°C for 300 s at the dew points of −35°C and −45°C. A Mn peak was observed at the steel surface at all soaking temperatures and dew points used in this study. The Mn oxide existed as external oxidation, and internal oxidation was not observed. At the dew points of both −35°C and −45°C, the peak of Mn was largest at 800°C.
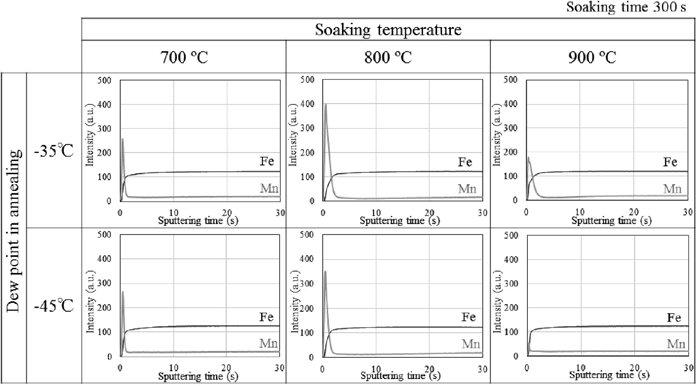
GDS depth profiles of samples annealed at 700°C, 800°C and 900°C for 300 s at dew points of −35°C and −45°C.
Figure 2 shows the effect of the soaking temperature on the amount of Mn oxidation measured by ICP-OES. After soaking for 300 s, the amount of Mn external oxidation was the largest at the soaking temperature of 800°C at both the −35°C and −45°C dew points. This result follows the GDS results. The amount of Mn oxidation increased in proportion to the square root of time at soaking temperatures ranging from 700°C to 900°C at the −35°C dew point and from 700°C to 800°C at the −45°C dew point. This result suggests that selective surface oxidation of Mn is a diffusion limited process of Mn diffusion from the interior to the surface of the steel, as also proposed in other reports.17,19) At the −45°C dew point, the oxidation amount did not change or decreased slightly with time at 850°C and 900°C.

Relation between external oxidation amount and square root of soaking time at dew points of −35°C and −45°C.18)
The proportionality coefficient of the relation between the amount of Mn oxidation and the square root of time was defined as the Mn oxidation rate constant, kp. Figure 3 shows the relation between the Mn oxidation rate constant, kp, and the soaking temperature. Because the amount of Mn oxidation did not change or decreased slightly with time at 850°C and 900°C at the −45°C dew point, kp of this temperature range was defined as 0.

Effect of soaking temperature on Mn oxidation rate constant, kp.18)
At the −35°C dew point, kp increased with soaking temperature, reached a peak value at 800°C and then decreased at higher temperatures. At the −45°C dew point, the peak value of kp was also observed at the soaking temperature of 800°C and decreased at temperatures higher than 800°C. The peak value of kp at the −45°C dew point was smaller than that at the −35°C dew point. These results show that the Mn oxidation rate decreased under lower dew point and higher soaking temperature conditions. According to diffusion theory, the diffusion coefficient increases exponentially with increasing temperature according to the Arrhenius equation, even at higher than 800°C. Thus, this phenomena cannot be explained only by diffusion theory.
3.2. Surface AnalysisIt was conjectured that the reason for the slowdown of the Mn oxidation rate at high temperature might be a difference in the oxide composition. Therefore, a sample surface analysis was carried out to clarify this point.
Figure 4 shows SEM images of the surface of the steel annealed at soaking temperatures of 700°C, 800°C and 900°C at the dew points of −35°C and −45°C. At 700°C, the amount of oxides observed in the SEM image was very small at both the −35°C and −45°C dew points. At 800°C, many particles of Mn external oxides with a size of about 0.1 μm were observed. The amount of particles was larger at −35°C than at −45°C. At 900°C, the amount of particles was smaller than that at 800°C. At the −35°C dew point, the particles of Mn oxides formed a linear pattern, suggesting that Mn diffuses through the steel grain boundaries and is oxidized preferentially on the grain boundaries. At −45°C, Mn oxides were hardly observed. These results concerning the amount of Mn oxides observed by SEM follow the results of GDS and ICP.

SEM images of surface of samples annealed for 300 s at soaking temperatures of 700°C, 800°C and 900°C and dew points of −35°C and −45°C.
Figure 5 shows the IR spectra of the surface of the steel annealed at the soaking temperatures of 700°C, 800°C and 900°C at the dew points of −35°C and −45°C. At all soaking temperatures and dew points, a peak was observed at around 514 cm−1, which derives from Mn oxide. No other peaks were observed. This result suggests that only Mn oxides are formed on the steel surface, and the soaking temperature and dew point do not affect the compounds of the oxide.

IR reflection spectra of samples annealed for 300 s at soaking temperatures of 700°C, 800°C and 900°C and dew points of −35°C and −45°C.
Figure 6 shows the XPS spectra of surface of the steel annealed at soaking temperatures of 700°C, 800°C and 900°C at the dew points of −35°C and −45°C. The peak derived from Mn–O was observed on the spectrum of Mn 2p at all temperatures and dew points. This result also suggests that only Mn oxide is formed on the annealed steel surface.

XPS spectra of samples annealed for 300 s at soaking temperatures of 700°C, 800°C and 900°C and dew points of −35°C and −45°C.
The Mn oxidation rate constant, kp was estimated by a diffusion equation and thermodynamic equilibrium in order to discuss the mechanism by which the measured kp decreased under the higher temperature and lower dew point conditions. From the results of IR and XPS, the composition of the oxide is assumed to be MnO. The formation reaction of MnO can be written as Eq. (1).
| (1) |
Figure 7 shows the Mn diffusion model in this study. The Mn concentration in the steel is constant as C0Mn at soaking time t=0, and at t>0, the steel is held in a constant atmosphere with a constant temperature, dew point and hydrogen partial pressure. The Mn concentration at the steel surface during annealing is kept as C1Mn. Here, the time spent in reaching the soaking temperature was considered to be 0 in this model, because in this experiment the amount of Mn external oxidation after annealing for a soaking time of 0 s was sufficiently small, even though the heating rate was 10°C·sec−1. This model can be described as follows by the diffusion coefficient, diffusion equation, initial condition and boundary condition.
| (2) |
| (3) |
| (4) |
| (5) |

Model of Mn concentration profile in steel soaked in an atmosphere for soaking time, t.
Here, D, D0, E0, R and T are the diffusion coefficient of Mn in steel, the temperature-independent pre-exponential of diffusion, the activation energy of diffusion, a gas constant and temperature, respectively. CMn(x,t), C0Mn and C1Mn represent the Mn concentration at depth position from steel surface x and soaking time t, the average Mn concentration in the steel before annealing and the Mn concentration at the steel surface during annealing, respectively. Equations (2) and (3) mean that Mn concentration in steel changes depending on Mn diffusion. Equation (4) means that the Mn concentration in the steel is constant as C0Mn at t=0. Equation (5) means that during annealing at t>0, the Mn concentration at the steel surface, i.e. x=0, is C1Mn.
Here, the thermodynamic equilibrium between the Mn concentration at the surface of the steel, C1Mn, and the atmosphere at the steel surface can be described as shown in Eq. (6) and can be translated into Eq. (7).
| (6) |
| (7) |
Here, pH2, pH2O, γMn, ΔG and ΔG° are the partial pressures of H2 and H2O, activation coefficient of Mn, Gibbs free energy and standard Gibbs free energy of Eq. (1), respectively.
The solution of diffusion Eq. (3) under the conditions of Eqs. (4) and (5) can be written as follows.20)
| (8) |
Here, erf represents an error function. According to Fick’s law, the flux of Mn at steel surface from the steel interior, J, can be written as follows.
| (9) |
Because all of the Mn that passes through the steel surface is consumed in Mn external oxidation in this case, the amount of Mn external oxidation, Δw at a time, t, is equal to the time integral of the flux of Mn at the steel surface. Δw can be written as follows using Eqs. (2), (7), (8) and (9)
| (10) |
Equation (10) shows that the amount of Mn consumed as external oxidation, Δw, is proportional to the square root of time. The proportionality coefficient corresponds to kp as shown in Eq. (11).
| (11) |
The values of γMn, D0 and E0 were estimated by comparing Eq. (11) and the measured kp of the results in Fig. 3. In this estimation, the literature data21) are used for ΔG° and values based on experimental conditions are used for the other parameters.
γMn was estimated based on the result that kp was nearly zero at the soaking temperature of 850°C and dew point of −45°C. From Eq. (11), kp is 0 at the soaking temperature of 850°C and dew point of −45°C when γMn is 0.25. As the activity coefficient of Mn in steel, values ranging from 0.1 to 1 have been reported.22,23) Thus, the value obtained in this study is within the range of the previous reports. Figure 8 shows the relation between the calculated values of the thermodynamic part of Eq. (11) and the soaking temperature. At lower soaking temperatures than 800°C at the dew point of −35°C and lower than 750°C at the dew point of −45°C, the thermodynamic part was nearly the same as
| (12) |
| (13) |

Relation between calculated values of thermodynamic part of kp and soaking temperature.
Figure 9 shows the Arrenius plot of the measured kp. According to Eq. (13), measured values of lnkp were proportional to the inverse of the temperature at lower temperatures than 800°C at the dew point of −35°C. D0 and E0 were calculated from the intercept and gradient of Fig. 9 as 2.9×10−4 m2·s−1 and 236 kJ·mol−1, respectively. The literature values of the diffusion coefficient of Mn in the α phase of steel bearing 1.9−3.6 mass%Mn are reported as D0: 3.5×10−4 m2·s−1 and E0: 241 kJ·mol−1.24) The values obtained in this study are nearly the same as these literature values.

Relation between lnkp and 1/T and fitting line.
Figure 10 shows the relation between kp calculated by the estimated γMn, D0 and E0, and the soaking temperature. The measured kp is also plotted on Fig. 10. The calculated kp was consistent with the calculated kp at both the −35°C and −45°C dew points.

Relation between calculated Mn oxidation rate constant kp and soaking temperature.
From the Eq. (11), the effects of the soaking temperature and dew point on kp can be explained as follows: The kp equation, Eq. (11), is a composite of the product of the thermodynamic part and the diffusion part. Because the thermodynamic part is a decreasing function to temperature as shown in Fig. 8 and the diffusion part is an increasing function to temperature, kp has an inflection point against temperature, which is determined by the balance of the thermodynamic part and the diffusion part. At low temperature, the thermodynamic part of the Eq. (11) approaches
The effect of the soaking temperature and dew point in annealing on Mn selective oxidation in Mn-containing steel was investigated. The results of the present study are summarized as follows.
(1) The amount of Mn selective oxidation increased in proportion to the square root of the soaking time. This result suggests that Mn selective oxidation is a diffusion limited process controlled by Mn diffusion in the steel.
(2) The selective oxidation rate of Mn increased as the temperature increased, reached a peak value at 800°C and then decreased at higher temperatures. This peak value depended on the dew point and was lower at the lower dew point.
(3) The Mn external oxidation rate was estimated by a diffusion equation and thermodynamic equilibrium, supposing that the formation of MnO is a diffusion limited process. At low temperatures, Mn external oxidation increases as the temperature increases because the diffusion coefficient increases. At high temperatures, the Mn external oxidation rate decreases because the gradient of the Mn concentration around the steel surface becomes small at temperatures near the equilibrium temperature of Mn/MnO. Considering the activity coefficient of Mn and the diffusion coefficient of Mn in steel, the calculated Mn external oxidation rate was consistent with the measured value.