2019 Volume 59 Issue 12 Pages 2327-2333
2019 Volume 59 Issue 12 Pages 2327-2333
The hydrogen embrittlement behavior of an ultra-high strength steel sheet consisting of ferrite and nanometer-sized precipitates has been investigated by a tensile test and sustained tensile-loading test. The amount of absorbed hydrogen of the present ferritic steel is significantly larger than that of the conventional martensitic steels. Hydrogen thermal desorption analysis indicates that a large amount of diffusible hydrogen exists and the nanometer-sized precipitates act as trap sites of hydrogen. In a tensile test in air after the saturation amount of hydrogen charging, the fracture strain decreases slightly. However, in a tensile test during hydrogen charging, the fracture strain decreases markedly despite a small amount of absorbed hydrogen, and the morphology of the fracture surface exhibits a unique brittle mode. Upon pre-straining, the saturation amount of hydrogen is more than doubled and the tensile properties deteriorate further. In the sustained tensile-loading test during hydrogen charging, no delayed fracture occurs even under high applied stress. No effects of hydrogen charging on stress relaxation are observed. The results of the present study imply that the increase in the hydrogen content enhances the degradation of tensile properties, but the hydrogen content is not necessarily an index of the hydrogen embrittlement of the ferritic steel. The dynamic interactions between hydrogen and deformation, and particularly, the continuous interactions during hydrogen charging, play important roles in hydrogen embrittlement.
The tensile strength of steel sheets for automotive structural parts is steadily increasing to satisfy governmental and corporate targets for weight reduction and crashworthiness. However, high strengthening of the steel sheet usually results in increasing susceptibility to hydrogen embrittlement, including delayed fracture. In ultra-high strength martensitic steel sheets having tensile strength of 1180 MPa or more, susceptibility to hydrogen embrittlement increases significantly and intergranular fracture related to the prior austenite grain boundary occurs.1,2,3,4,5,6,7) Even in dual phase steel sheets including almost no prior austenite grain boundary, crack nucleation is dominated by the characteristics of martensite itself.8,9,10) It is likely that the presence of martensite substantially increases the susceptibility to hydrogen embrittlement of ultra-high strength steel sheets.
Susceptibility to hydrogen embrittlement has been improved by introducing strong hydrogen trapping precipitates.11,12,13,14,15) In particular, nanometer-sized (Ti, Mo)C in tempered lath martensitic steel increases resistance to hydrogen embrittlement.15) Here, if a ferritic steel contains no martensite with a high susceptibility to hydrogen embrittlement and has a large number of hydrogen trapping nanometer-sized precipitates, its hydrogen embrittlement behavior will be different from that of martensitic steel.
Hydrogen embrittlement behavior should be evaluated by not only static tests such as a sustained tensile-loading test, but also dynamic tests such as the tensile test, in particular, if even its fundamental behavior is unknown. In the tensile test, the dynamic interactions between hydrogen and deformation often markedly affect hydrogen embrittlement.4,15,16,17,18,19,20) These interactions enhance the nucleation of defects.4,15,16,17,18) Ferritic steel is suitable for evaluation of the effects of the interactions on hydrogen embrittlement because of its low density of initial defects.
The purpose of the present study is to investigate the hydrogen embrittlement behavior of an ultra-high strength ferritic steel sheet with nanometer-sized precipitates by tensile tests after and during hydrogen charging. In addition, the sustained tensile-loading test and tensile-straining test during hydrogen charging were conducted to investigate the delayed fracture properties.
The chemical composition of the ferritic steel containing nanometer-sized precipitates used in the present study is shown in Table 1. Titanium, molybdenum and vanadium were added at 0.18, 0.34 and 0.42 mass%, respectively. Hot-rolled steel sheet with the thickness of 1.4 mm was prepared. The finishing temperature of hot-rolling was 890°C and coiled at 560°C. Subsequently, the top and bottom surfaces were machine-ground to a thickness of 1.2 mm. Scanning electron microscope (SEM) images of the microstructure etched with a 3% nital solution are shown in Fig. 1(a). The grain size of the ferrite was approximately 2 μm. Transmission electron microscope (TEM) images of the specimen are shown in Fig. 1(b). Thin foils for TEM observation were prepared by twin-jet electropolishing in an 8% HClO4 + 82% C2H5OH aqueous solution. The arrows in the figure indicate the nanometer-sized precipitates in the ferritic steel. These precipitates are carbides.21)
| C | Si | Mn | P | S | Al | N | Others | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.18 | 0.01 | 1.3 | 0.01 | 0.001 | 0.04 | 0.005 | Ti, Mo, V | Bal. |

(a) SEM image and (b) TEM image of ferritic steel containing nanometer-sized precipitates. The arrows indicate nanometer-sized precipitates.
For the tensile test, the specimens were machined parallel to the rolling direction with a thickness of 1.2 mm, gauge length of 15 mm and gauge width of 6 mm, as reported previously.8,10) The tensile tests were carried out at a strain rate of 8.33 × 10−4 s−1 at room temperature (25 ± 2°C). The tensile properties of the specimens are summarized in Table 2. The highest strength during the tensile test was at the upper yield point (1191 ± 10 MPa). After yielding, the increase in strength due to work hardening was very small; as a result, the tensile strength which indicates the limit strength of uniform elongation was 1173 ± 10 MPa, and then necking and fracture occurred. Owing to the single ferrite phase, total elongation (17 ± 1%) was larger than that of the martensitic steel of the same strength grade in the previous report.8)
| Yield stress (MPa) | Tensile stress (MPa) | Elongation (%) |
|---|---|---|
| 1191 ± 10 | 1173 ± 10 | 17 ± 1 |
Hydrogen embrittlement behavior was evaluated by tensile tests at strain rates of 8.33 × 10−4 s−1 or 8.33 × 10−6 s−1 after and during hydrogen charging, and delayed fracture properties were investigated by the sustained tensile-loading test and tensile-straining test during hydrogen charging using specimens of the same size as the tensile test specimen. Hydrogen charging was performed by cathodic electrolysis using a 3% NaCl + 0.3% NH4SCN aqueous solution at room temperature with a current density of 50 A/m2. The fracture surfaces were observed by SEM. The sustained tensile-loading test and sustained tensile-straining test, i.e., stress relaxation test, during hydrogen charging were carried out under a high applied stress (1000 to 1100 MPa). The hydrogen charging under the above-mentioned conditions was started after applying stress. The time to fracture of the specimens was measured until 100 h.
To investigate the effects of pre-deformation on hydrogen embrittlement behavior, the specimens were subjected to the applied strain of 0.10 in air before the tensile tests and the sustained tensile-loading test. These tensile tests were conducted at the strain rate of 8.33 × 10−6 s−1.
Hydrogen thermal desorption analysis (TDA) was performed using a gas chromatograph at a constant heating rate of 100°C/h from room temperature to 400°C, and the sample was analyzed at 5 min intervals using Ar as the carrier gas. The specimens were cut at both ends and subjected to ultrasonic cleaning with acetone for 5 min. Subsequently, the specimens were dried in ambient air and then subjected to TDA, which was started 15 min after removal of the specimens from the test solutions. The amount of desorbed hydrogen was defined as the integrated peak intensity.
Figure 2 shows the TDA curves of the specimens subjected to hydrogen charging without applied stress for 30 min, 24 h and 48 h. In addition, this figure also shows the curve of aging at room temperature for 24 h in air after hydrogen charging for 24 h. The hydrogen desorption of the uncharged specimen was the limit of detection. The amount of desorbed hydrogen, i.e., hydrogen absorbed during hydrogen charging, for the present ferritic steel after hydrogen charging even for 30 min (4.13 mass ppm) was larger than that for the martensitic steel after hydrogen charging for 24 h (1.13 mass ppm).8) Under hydrogen charging for 24 h, the amount of desorbed hydrogen for the ferritic steel (8.00 mass ppm) was approximately 7 times larger than that of the martensitic steel.8) The amount of desorbed hydrogen after hydrogen charging for 48 h was 7.19 mass ppm. This indicates that hydrogen absorption saturates after 24 h. Upon aging in air for 24 h after hydrogen charging for 24 h, the amount of desorbed hydrogen was 3.61 mass ppm; thus, the amount of hydrogen decreased by approximately one-half. Hydrogen desorption was scarcely detected in the low temperature range, whereas hydrogen desorption in the high temperature range decreased only slightly. This result indicates that the hydrogen desorption in the low temperature range is related to diffusible hydrogen. In contrast, hydrogen desorption in the high temperature range appears to be related to the nanometer-sized precipitates contained in the steel, because hydrogen is strongly trapped at the precipitates.11,12,13,14,15,22,23,24) Before aging, the desorption peaks at lower temperatures were observed from 25°C to 40°C, whereas the desorption peaks at higher temperatures were observed from 40°C to 200°C. In the case of martensitic steel, the peaks at the lower temperatures were from 25°C to 40°C, whereas the peaks at the higher temperatures were from 40°C to 100°C.8) In high strength martensitic steels containing nanometer-sized (Ti, Mo)C precipitates, hydrogen is trapped at the nanometer-sized precipitates, thereby absorbing a large amount of hydrogen.15) In the ferritic steel investigated in this report, hydrogen is presumably also trapped at the nanometer-sized precipitates in the same manner as in the martensitic steel.

Hydrogen thermal desorption curves of ferritic steel containing nanometer-sized precipitates after hydrogen charging.
Figure 3(a) shows the stress-strain curves at a strain rate of 8.33 × 10−4 s−1 for various hydrogen charging conditions. The fracture strain of the specimen subjected to the tensile test in air after hydrogen charging (pre-charging) for 48 h was slightly lower than that without hydrogen charging. This result suggests that the hydrogen embrittlement susceptibility of the ferritic steel is very low. In the tensile test during hydrogen charging, the fracture strain markedly decreased. In this case, the amount of charged hydrogen was as much as approximately 2 mass ppm, even though the elapsed time of the tensile test, i.e., charging time, was at most 3 min. Considering the fact that hydrogen absorption is enhanced by hydrogen charging during deformation in a Ni–Ti superelastic alloy,19) the hydrogen absorption rate should increase due to dynamic plastic deformation. Nonetheless, even when the hydrogen absorption rate increased, the amount of absorbed hydrogen did not reach the saturation amount (7 to 8 mass ppm) before fracture. Furthermore, after pre-charging for 48 h followed by the tensile test during hydrogen charging, fracture occurred with almost no necking. The stress-strain curves at a slow strain rate of 8.33 × 10−6 s−1 are shown in Fig. 3(b). The effects of the slow strain rate on tensile behavior were only slightly observed in air after hydrogen charging for 48 h. Thus, the strain rate has almost no effect on the interactions between hydrogen and deformation. In contrast, in the tensile test during hydrogen charging, while fracture occurred with only slight plastic deformation and the elapsed time of the test was around 3 h, the amount of absorbed hydrogen was larger than 8 mass ppm. This degradation of tensile properties appears to be associated primarily with the increase in the hydrogen content rather than by the slow strain rate. The mechanism of degradation of tensile properties is considered to be enhancement of defect nucleation induced by the interactions between hydrogen and deformation.16,17) The degradation of tensile properties also appears to be caused by these interactions in the present study. In addition, the results clearly indicate that hydrogen charging during the tensile tests markedly degrades tensile properties. To explain this marked degradation, we propose that continuous interactions between hydrogen and deformation play essential roles in the degradation of tensile properties. Specifically, the dynamic interactions between hydrogen and plastic deformation, not only substantially enhance defect nucleation such as vacancy, but also cause the hydrogen state to change from diffusible hydrogen to hydrogen trapped at defects.17) Compared with trapped hydrogen, diffusible hydrogen interacts strongly with plastic deformation.17,25) Hence, it should be noted that the marked degradation observed in these tests results from continuous interactions due to hydrogen charging during deformation. In the tensile test after hydrogen charging, it is likely that since most of the diffusible hydrogen is trapped at defects by the early stage of dynamic interactions, the deterioration of mechanical properties is small. As discussed in the following, the simple hydrogen content is not necessarily a critical index of hydrogen embrittlement. It is necessary to evaluate by the hydrogen content interacting with the deformation.

Engineering stress-strain curves under various hydrogen charging conditions at strain rate of (a) 8.33 × 10−4 s−1 and (b) 8.33 × 10−6 s−1.
The fracture surfaces corresponding to the stress-strain curves (Fig. 3(a)) are shown in Fig. 4. In the tensile test in air without hydrogen charging, the fracture surface was composed of large primary and small secondary dimples; i.e., it displayed a ductile fracture mode (Fig. 4(a)). In the tensile test in air after hydrogen charging for 48 h, the dimples became very shallow (Fig. 4(b)) and secondary dimples were rarely observed. Thus, it appears that the effects of hydrogen on the fracture process are more apparent than those on the stress-strain curve. In the tensile test during hydrogen charging without (Fig. 4(c)) or with pre-charging for 48 h (Fig. 4(d)), the brittle fracture area (fracture initiation area) on the fracture surfaces exhibited a unique brittle morphology, which is not classified into the conventional intergranular fracture or quasi-cleavage. This unique morphology was characterized as flake-like without dimples. In general, cleavage facets are almost the size of ferrite grains; however, the flake size did not always correspond to the size of the ferrite grains and depended on test conditions. Thus, it is unlikely that this unique fracture mode is associated with the cleavage fracture mode. Dimples were observed except in the brittle fracture area.

SEM images of typical fracture surface of specimens subjected to tensile test (a) in air, (b) in air after hydrogen pre-charging for 48 h, (c) during hydrogen charging and (d) during hydrogen charging after hydrogen pre-charging for 48 h.
To investigate the effects of the hydrogen state, i.e., diffusible hydrogen or trapped hydrogen, on the degradation of tensile properties, the specimen was aged at room temperature in air for 24 h after hydrogen charging for 24 h and was then subjected to a tensile test in air, as shown in Fig. 5. Although the slight decrease of tensile strength might be experimental error, ductility was almost completely recovered by aging despite the existence of residual hydrogen. As shown in Fig. 2, the amount of desorbed hydrogen of the specimen was decreased from 8.00 to 3.61 mass ppm by aging, particularly in the low temperature range. However, in the tensile test during hydrogen charging, the decrease of ductility was larger, as shown in Fig. 3, even though the amount of absorbed hydrogen (approximately 2 mass ppm) was lower than 3.61 mass ppm. Therefore, the total amount of hydrogen is not necessarily an index of hydrogen embrittlement. In the hydrogen desorption curve of the specimen after hydrogen charging for 30 min (Fig. 2), the peaks at the higher temperatures were almost the same as that of the aged specimen, even though hydrogen desorption was observed at the lower temperatures. The hydrogen which is desorbed near room temperature is presumably completely desorbed by aging in air for 24 h. Thus, the hydrogen desorbed at the lower temperatures is probably associated with the decrease in ductility. Because the hydrogen desorbed at the higher temperatures is related to hydrogen trapped at defects such as nanometer-sized precipitates, it does not appear to have a substantial effect on the plastic deformation process. Thus, it is unlikely that the nanometer-sized precipitates play a crucial role in hydrogen embrittlement, in spite of the fact that they increase the saturation amount of absorbed hydrogen. Thus, the decrease in ductility in the tensile test during hydrogen charging should not be explained by only the hydrogen content.

Effects of aging after hydrogen charging on engineering stress-strain curves in air at strain rate of 8.33 × 10−4 s−1.
Figure 6 shows the thermal desorption curve of the 0.10 pre-strained specimen subjected to hydrogen charging for 48 h without applied stress. The amount of desorbed (absorbed) hydrogen (17.61 mass ppm) more than doubled as a result of pre-straining. The amount of desorbed hydrogen increased at the higher temperatures range from 40°C to 200°C as well as at the lower temperatures range from 25°C to 40°C. For an interstitial free mild steel sheet, Nagumo et al.26) reported that the amount of desorbed hydrogen increases due to pre-straining and the desorption peak at around 50°C increases. At around 50°C, hydrogen is detrapped from dislocations, and the hydrogen trapped at vacancy clusters is desorbed as a result of the annihilation of the vacancy clusters.26) Choo et al.27) reported that the amount of absorbed hydrogen increases with increasing dislocation density in pure iron. On the basis of the previous reports,26,27) the present results indicate that upon pre-straining, the densities of dislocations and vacancy clusters increase, thereby greatly increasing the amount of hydrogen desorbed at higher temperatures. To clarify the reason for the increase in the amount of hydrogen desorbed at the lower temperatures, i.e., diffusible hydrogen, further investigation is needed.
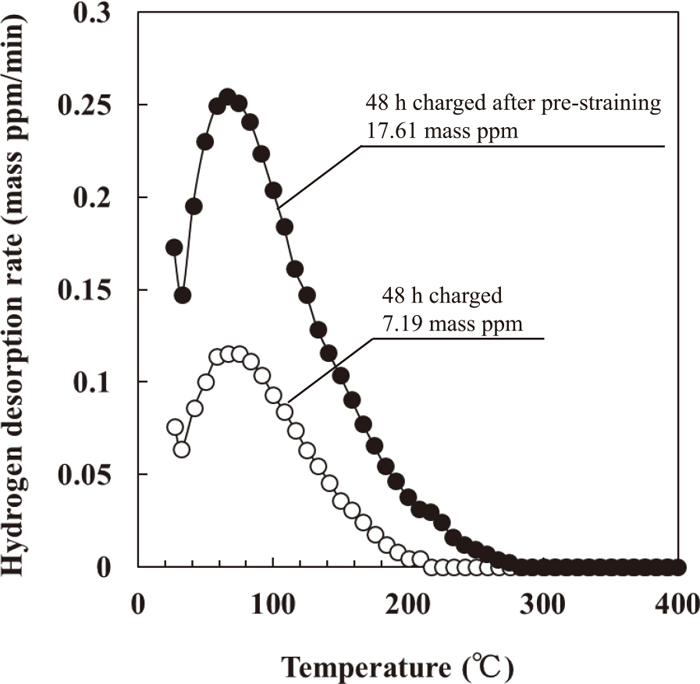
Hydrogen thermal desorption curves of specimen subjected to pre-straining followed by hydrogen charging.
Figure 7 shows the results of the 0.10 pre-strained specimens subjected to the tensile test at the strain rate of 8.33 × 10−6 s−1 in air or during hydrogen charging after pre-charging for 48 h. At the beginning of the tensile test, the amount of absorbed hydrogen was approximately 18 mass ppm, as shown in Fig. 6. In the tensile test in air, the fracture strain of the pre-stained specimen decreased significantly. During hydrogen charging (approximately 100 min), the pre-strained specimen subjected to the tensile test fractured in the elastic deformation region. This experimental condition greatly deteriorates the tensile properties. The degradation of these tensile properties can be attributed to the increase in the amount of hydrogen desorbed at the lower temperatures rather than that at the higher temperatures, as shown in Fig. 6.

Engineering stress-strain curves at strain rate of 8.33 × 10−6 s−1 in air or during hydrogen charging for specimen subjected to hydrogen pre-charging for 48 h after 0.10 pre-straining.
The brittle-like areas on the fracture surfaces of the 0.10 pre-strained specimens are shown in Fig. 8. In the tensile test in air after hydrogen charging for 48 h (Fig. 8(a)), although small dimples surrounded the flat facet, large primary dimples were scarcely observed. This brittle-like fracture was explained by the increasing amount of absorbed hydrogen due to pre-straining. However, no unique brittle fracture (flake-like) without dimples, which was observed in the tensile test during hydrogen charging (Figs. 4(c) and 4(d)), was confirmed. This unique brittle fracture was observed on the fracture surface of the pre-strained specimen that fractured during hydrogen charging (Fig. 8(b)), implying that the unique brittle mode must result from the continuous interactions between hydrogen and deformation rather than the hydrogen content. The amount of absorbed hydrogen induced by pre-straining appears to considerably enhance hydrogen embrittlement.

SEM images of typical fracture surface under tensile tests (a) in air and (b) during hydrogen charging for specimen subjected to hydrogen pre-charging for 48 h after 0.10 pre-straining.
The results of the sustained tensile-loading test during hydrogen charging are plotted in Fig. 9 for the specimens with and without 0.10 pre-straining. The arrows in the figure denote the result for a non-fractured specimen; thus, the time to fracture was longer than 100 h. For comparison, the results of the martensitic steel (carbon content of 0.13 mass%) tested under the same conditions8) are also shown in Fig. 9. The specimen size was the same as that of the ferritic steel tested in the present study. The 0.2% proof strength and the tensile strength were 1146 MPa and 1253 MPa.8) It should be noted that the ferritic steel containing nanometer-sized precipitates did not fracture even under the stress of 1100 MPa. Since the martensitic steel fractured in approximately 9 h under the same conditions, the ferritic steel presumably has extremely low susceptibility to delayed fracture in the sustained tensile-loading test. Furthermore, even for the 0.10 pre-strained specimen, no delayed fracture occurred under the applied stress of 1050 MPa. It is most likely that the ferritic steel containing nanometer-sized precipitates has superior resistance to delayed fracture. As reported by Nagao et al.,15) one of the factors of superior hydrogen embrittlement behavior appears to be that fine precipitates act as hydrogen trap sites. In dual phase steels consisting of ferrite and martensite,10) cracks do not nucleate in the ferrite, although numerous cracks are observed in the martensite under the sustained tensile-loading test during hydrogen charging. In addition, ferrite displays the superior resistance to crack propagation.10) Similarly, the ferrite matrix probably suppresses crack nucleation and propagation in the sustained tensile-loading test, thereby resisting delayed fracture.

Time to fracture versus applied stress of ferritic steel containing nanometer-sized precipitates and martensitic steel in sustained tensile-loading test during hydrogen charging.
The results of the sustained tensile-straining test, i.e., stress relaxation test, in air and during hydrogen charging are shown in Fig. 10. For comparison, the results of the above-mentioned martensitic steel are also shown. For the ferritic steel, no effects of hydrogen charging on stress relaxation were observed, but with the martensitic steel, hydrogen rapidly enhanced stress relaxation and fracture. The fact that no fracture occurred under static applied stress or strain clearly supports the conclusion that hydrogen embrittlement of ferritic steel results from the dynamic interactions between hydrogen and plastic deformation.

Changes in stress of ferritic steel containing nanometer-sized precipitates and martensitic steel under sustained tensile-straining test in air or during hydrogen charging.
In a study of an ultra-high strength steel sheet consisting of ferrite and nanometer-sized precipitates, we have demonstrated that hydrogen embrittlement is markedly induced by hydrogen charging during deformation by performing tensile tests after and during hydrogen charging, and sustained tensile-loading tests and tensile-straining tests during hydrogen charging.
• In the present steel, the amount of absorbed hydrogen is significantly larger than that of the conventional martensitic steel. The nanometer-sized precipitates probably act as hydrogen trap sites.
• In the tensile test in air after hydrogen charging, marked degradation of tensile properties is only slightly observed despite the large amount of hydrogen. However, in the tensile test during hydrogen charging, although the amount of charged hydrogen is small, tensile strain decreased markedly and a unique brittle fracture surface is observed. Hydrogen pre-charging or pre-straining considerably enhances the degradation of tensile properties.
• In the sustained tensile-loading test during hydrogen charging, no fracture occurs even under the condition of high applied stress and pre-straining. In the sustained tensile-straining test during hydrogen charging, no effects of hydrogen on stress relaxation behavior are observed.
• The results under static applied stress and strain indicate superior delayed fracture resistance. Simultaneously, this finding implies that the dynamic interactions between hydrogen and deformation during the tensile test, and particularly, the continuous interactions during hydrogen charging are essential for hydrogen embrittlement.