2019 Volume 59 Issue 4 Pages 672-678
2019 Volume 59 Issue 4 Pages 672-678
Carbothermic reduction of FeS in the presence of CaO was experimentally studied by employing a multi-mode microwave generator at 1050 W and 2.45 GHz as an external heat source to mitigate CO2 and SO2 emissions. According to XRD analysis, an ion exchange reaction between FeS and CaO was initiated at temperatures lower than 645°C and progressed by a further heating to 850°C without any evidence for the onset of a reduction reaction. Detection of Fe phase in the XRD pattern of sample heated to 920°C and existence of CO/CO2 in the off-gas during microwave treatment demonstrated that the reduction reaction initiates at ca. 850°C. It was found that the onset of reduction reaction promotes the ion exchange reaction by FeO consumption via reduction to Fe. Moreover, optical microscope and SEM-EDX observations showed that carbon can be absorbed by metallic iron to make molten iron particles at 1290°C.
Carbothermic reduction of iron sulfide cannot perform in the absence of a desulfurizer due to the thermodynamic reasons.1) Lime (CaO) has a high tendency for reaction with sulfur to form CaSO4 and CaS in the oxidizing and reducing atmosphere, respectively.2,3) Hara et al.3) reported the production of Cu, Co and Fe after a carbothermic reduction of sulfide concentrate containing copper, cobalt, and iron in the presence of CaO at 1000°C. Also, Hara et al.4) has studied the reduction of a mineral sulfide-CaO-C mixture and demonstrated that an increase in mole ratio of C:CaO improves magnetic separation of products into metal and CaS.
The carbothermic reduction of FeS in the presence of lime can be considered as a two-stage reaction;2,5) desulfurization reaction, Eq. (1), and reduction reaction, Eqs. (2) and/or (3). The total reaction is represented by Eq. (4).
| (1) |
| (2) |
| (3) |
| (4) |
Considering the thermodynamic information presented in Eqs. (1), (2), (3), (4), the desulfurization reaction is an exothermic reaction (ΔH<0) which can proceed after providing the energy required for activation of this reaction whereas the reduction reaction is an endothermic reaction (ΔH>0) which requires energy in form of heat to progress. The heat provided by exothermic desulfurization reaction is insufficient to initiate the reduction reaction regarding the total reaction, Eq. (4), which is also endothermic (ΔH>0). Therefore, an external heat source is required to generate a sufficient heat for the onset of both desulfurization and reduction reactions.
Recently, microwave heating is applied as a new heating method in order to eliminate CO2 emission during treatment of powder materials.6,7) Carbon, as a good microwave susceptor, plays a vital role in microwave absorption. Such extraordinary characteristic of carbon has prompted research into the carbothermic reduction of iron oxides under microwave irradiation.8,9,10,11,12,13,14) It is reported that the carbothermic reduction of iron oxide during microwave heating requires a shorter reaction time and a lower temperature than that in the conventional heating methods.9,15) Such advantages are attributed to the specific characteristics of microwave heating such as rapid, selective, and volumetric heating.16,17,18,19)
On the other hand, higher stability of the metal oxide than metal sulfide demonstrates a greater reactivity of mineral sulfide in an ion exchange reaction.2) For example, the Gibbs free energy of the ion exchange reaction between FeS and CaO is negative indicating higher stability of the FeO than FeS. Furthermore, metallization of Fe is restricted by the reduction reaction owing to a higher stability of FeO than metallic iron.2) Kutsovskaya et al.20) studied the reduction reaction in a mixture of FeS:CaO:C = 1:1.1:1.1 and showed that a reduction of 70% attains in 120 min at 950°C and that of 95% in 30 min at 1050°C. Hara2) reported that the rate of reduction reaction should be higher than desulfurization reaction to prevent SO2 emission which caused by a reaction between metal oxide and mineral sulfide. Therefore, C/CaO ratio should be greater than 1 during carbothermic reduction of mineral sulfides in the presence of CaO.
In the present study, carbothermic reduction of FeS in the presence of CaO is investigated by microwave heating of samples containing 1:1:2 stoichiometric mole ratio of FeS:CaO:C to mitigate CO2 and SO2 emissions during metallic iron production via desulfurization and reduction reactions.
Reagent CaCO3 powder (purity, 99.5%) was calcined at 1300°C for 600 min followed by crushing and grinding to the grain size of less than 45 μm. Formation of CaO after calcination process was confirmed by X-ray diffraction analysis; however, small amount of CaCO3 possibly remain which cannot be detected in the XRD pattern. Reagent FeS (purity, 99%) was crushed and ground to the grain size of less than 45 μm. Reagent graphite powder (purity, 99%, grain size, 75 μm) and prepared CaO and FeS were mixed well to prepare a mixture powder containing 1:1:2 stoichiometric mole ratio of FeS:CaO:C. To prepare the briquette samples, approximately 0.02 g of a 5 mass% water solution of polyvinyl alcohol was added as a binder to 3 g of the mixture powder. A cylindrical briquette sample (15 mm diameter, 8 mm high) was formed by a cold-press followed by drying at 120°C for 600 min. Prepared briquette samples were subjected to microwave heating using a multi-mode microwave generator with a maximum output power of 1.5 kW at 2.45 GHz under N2 (1 L/min) atmosphere in which both the perpendicular magnetic, H, and electric, E, fields contribute to heating, as shown in Fig. 1. A constant power of 1050 W was manually set until the end of heating in all experiments using three tuning stubs.
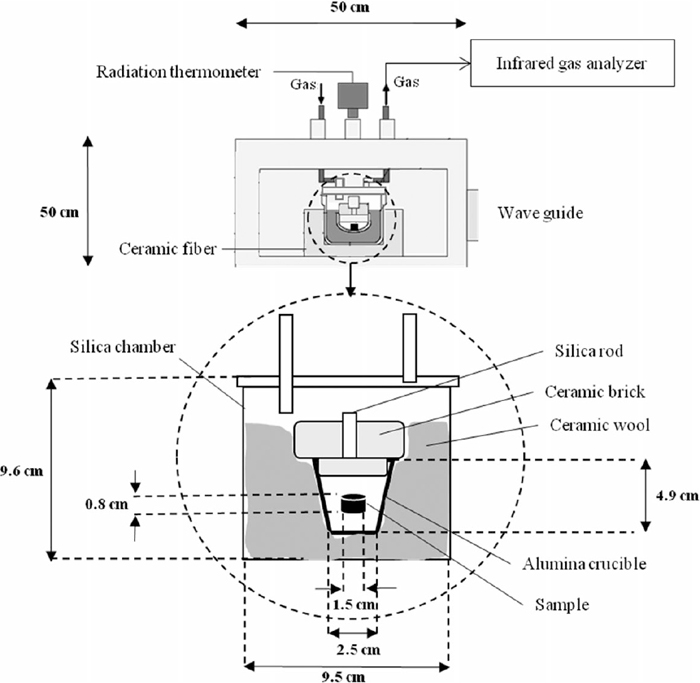
Schematic set-up for MW heating.
The sample was contained in an alumina crucible (41 mm OD, 36.5 mm ID, 49 mm high) which surrounded by ceramic wool and covered by an insulation ceramic brick. The crucible was placed in a silica chamber (95 mm OD, 85 mm ID, 96 mm high) where the sample was protected from oxidation by flowing N2 during the experiments. The ceramic wool and fiber were served as a thermal insulator to prevent heat loss during the heating and also used as a protector for silica chamber. The generated microwaves irradiated through the wave guide into the microwave box wherein the silica chamber was installed.21) Sample’s temperature was measured through a window above the sample using an infrared radiation thermometer. The measurement temperature range of the thermometer is 330–1500°C. To identify the phase transformations during heat treatment, microwave irradiation was stopped after 2, 10, 25, 30, and 35 min, then treated samples were subjected to phase analysis using X-ray diffraction (XRD, Cu-Kα; λ = 1.54 Å; scan speed, 51.9°/min; power, 3 kW; RIGAKU Smartlab, ZOTK, JAPAN). For this purpose, a portion of the treated sample was ground in a ceramic mortar and pestle. Further, XRD internal quantitative analysis method was applied to study reaction progress by quantitatively evaluation of final products after a certain treatment duration. For this purpose, 10 mass% reagent Si powder (purity, 99%, grain size, 75 μm) was added as standard material to each sample then ICaS/ISi and IC/ISi were calculated after X-ray diffraction analysis of samples.
The microstructure of the remaining part of treated samples was analysed using optical microscope observation. Moreover, carbon and sulfur contents of the sample were measured before and after treatment using a carbon/sulfur analyzer (HORIBA Scientific EMIA-320V2 Carbon-Sulfur Analyzer). Also, amount of CO and CO2 gases generated during microwave treatment were measured using an infrared off-gas analyzer (URA-208, SHIMADZU). To evaluate reaction progress, weight change ratio was calculated by dividing the weight change of the sample by its initial weight.
Figure 2 shows the average temperature of samples heated via microwave irradiation for 2, 10, 25, 30, and 35 min with the standard deviation represented as error bars. A similar temperature profile was observed in all samples, confirming the reproducibility of the method.
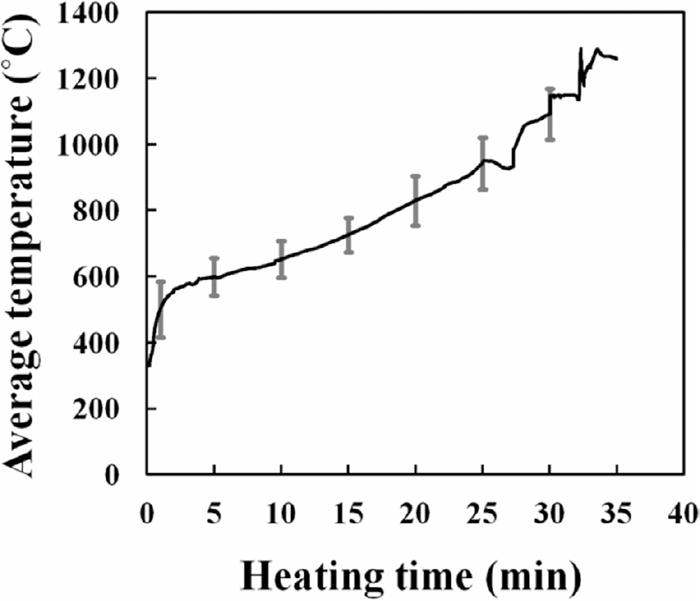
Average temperature of samples heated via microwave irradiation for 2, 10, 25, 30, and 35 min. Error bars represent the standard deviations of the average temperature.
A gradual increase in temperature was observed during microwave heating, which would be due to the presence of carbonaceous materials as a microwave susceptor in all samples. Such heating behaviour was also observed in previous studies.9,11,21,22)
Figure 3 illustrates XRD patterns of samples heated to 520, 645, 920, 1040, and 1290°C in 2, 10, 25, 30, and 35 min, respectively.
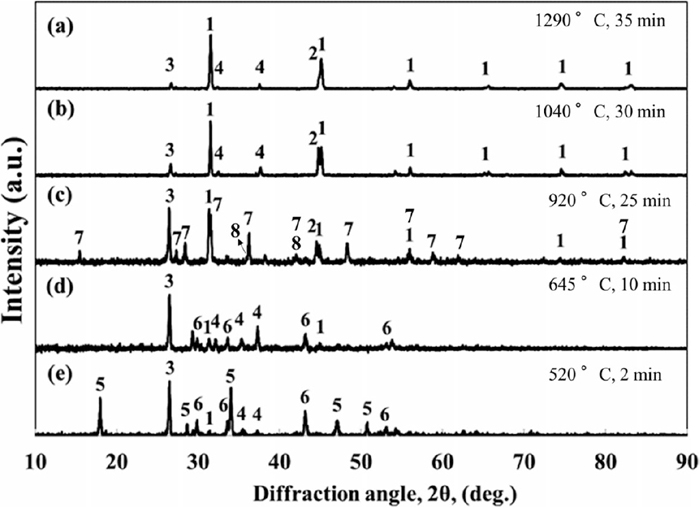
XRD patterns of samples heated to (a) 1290°C in 35 min, (b) 1040°C in 30 min, (c) 920°C in 25 min, (d) 645°C in 10 min, (e) 520°C in 2 min. 1: CaS, 2: Fe, 3: C, 4: CaO, 5: Ca(OH)2, 6: FeS, 7: FeO·CaS, 8: FeO.
Detecting Ca(OH)2 phase in the pattern of sample heated to 520°C in 2 min, see Fig. 3(e), indicates that the sample was hydrated according to Eq. (5) before microwave processing, although the CaO powder was kept in a vacuum chamber to protect it from hydration. Therefore, formation of Ca(OH)2 before microwave treatment is likely attributed to the hydration of a small portion of CaO in the mixture powder via a reaction with aqueous solution polyvinyl alcohol (the binder) according to Eq. (5).
| (5) |
In addition, the desulfurization reaction, Eq. (1), was initiated in sample heated to 520°C regarding a weak CaS peak detected in the XRD pattern of this sample, see Fig. 3(e).
XRD pattern of sample treated for 10 min, see Fig. 3(d), demonstrated that a further heating to 645°C causes fully dehydration of the sample at temperatures lower than 645°C. The absence of iron oxide peaks despite detection of weak CaS peaks in the XRD pattern of samples heated to 520 and 645°C will be explained at the end of section 3.1.
Presence of Fe phase in the XRD pattern of sample heated to 920°C, see Fig. 3(c), demonstrated the onset of reduction reaction, Eqs. (2) and/or (3), at 645–920°C. Further, a significant increase in the intensity of CaS peak at 31° was observed in the XRD pattern of sample heated to 920°C, see Fig. 3(c), compared to sample heated to 645°C, see Fig. 3(d). This indicates an extraordinary progress of desulfurization reaction via Eq. (1) in the former sample that is attributed to the consumption of FeO (as a product of the desulfurization reaction, Eq. (1)) via reduction to Fe at 645–920°C, which increases the driving force for a further progress in Eq. (1). On the other hand, an oxysulfide phase (FeO∙CaS) was detected in the XRD pattern of sample heated to 920°C, Fig. 3(c). This phase is considered as an intermediate phase during desulfurization reaction and its formation confirms the progress of this reaction. Formation of oxysulfide phases during carbothermic reduction of sulfide minerals in the presence of lime is also reported in previous studies.1,2,23)
In addition, formation of the intermediate oxysulfide phase was confirmed by SEM-EDX analysis of samples heated to 645 and 920°C, as shown in Figs. 4(a) and 4(b), respectively; however, no oxysulfide phase was detected in the XRD pattern of sample heated to 645°C owing to the small amount of this phase.
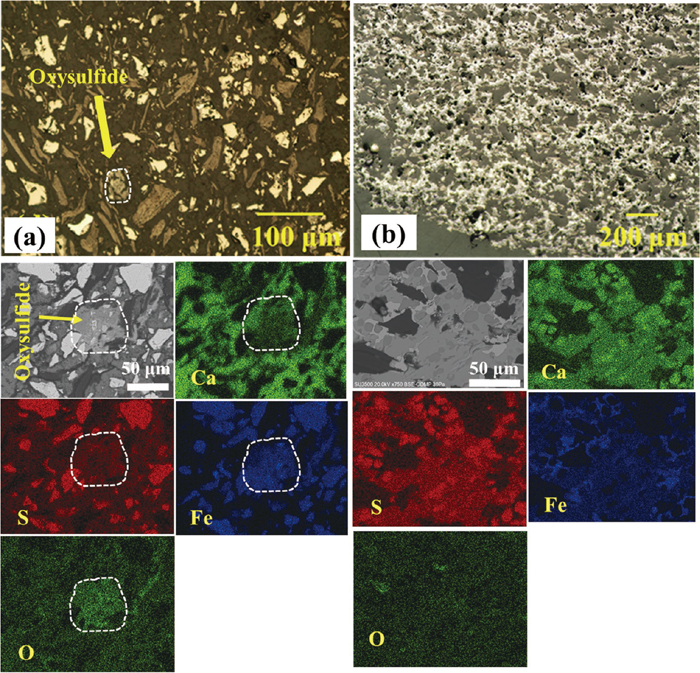
Optical microscope and SEM-EDX images of samples heated to (a) 645°C in 10 min, and (b) 920°C in 25 min via microwave irradiation.
The intermediate phase was transformed to final products (CaS and Fe) after a further heating to 1040°C, see Fig. 3(b), and no more phase change was observed at higher temperatures, Fig. 3(a).
Weight change ratio of treated samples for 2, 10, 25, 30, and 35 min was calculated according to Eq. (6) to confirm the progress in desulfurization and reduction reactions via phase transformations as mentioned above.
| (6) |
Where ΔWt (dimensionless) is the total weight change ratio after treatment for t min, Wi (g) is the initial weight of sample, and Wt (g) is the weight of sample after treatment for t min.
Figure 5 shows carbon and sulfur contents and weight change ratio of samples heated for 2, 10, 25, 30, and 35 min during microwave treatment.
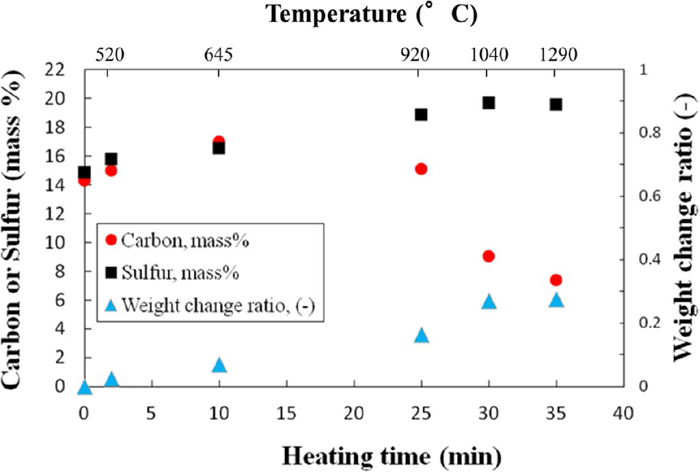
Carbon and sulfur contents and weight change ratio of samples heated for 2, 10, 25, 30, and 35 min. (Online version in color.)
As shown in Fig. 5, a decrease in weight of the sample was observed after 2 min treatment whereas the absence of Fe phase in the XRD pattern of this sample, see Fig. 3(e), indicates that the reduction reaction, Eq. (2), cannot initiate at 520°C. Therefore, the weight change of the sample at ca. 520°C is attributed to either removal of volatile materials (the binder) or dehydration reaction regarding the Gibbs free energy of Eq. (5) obtained by FactSage® software. Accordingly, such weight loss is the cause of the increase in carbon and sulfur fraction of the mixture in the beginning of the treatment, as shown in Fig. 5.
The absence of Fe phase in XRD pattern of sample heated to 645°C, indicates that the reduction reaction, Eqs. (2) and/or (3), cannot initiate at temperatures lower than 645°C in the present study. Such behavior is in good agreement with the results of Marina et al.20) and Rosenqvist24) who also reported that the iron production from a mixture of FeS, CaO, and C is thermodynamically feasible above 650 and 730°C, respectively. Therefore, the weight loss in sample heated to 645°C in 10 min is attributed to either the fully dehydration reaction, as confirmed by XRD analysis (Fig. 3(d)), or removing of volatile materials such as binder. The absence of Ca(OH)2 in the XRD pattern of sample heated to 645°C (Fig. 3(d)) in 10 min indicates that the calcium hydroxide has no effect on overall reaction at temperatures >645°C.
Further weight loss in sample heated to 920°C in 25 min accompanied by a decrease in carbon contents of this sample is attributed to the onset of reduction reaction at 645–920°C according to Eqs. (2) and/or (3), as confirmed by detecting of Fe in XRD pattern of sample heated to 920°C in 25 min, see Fig. 3(c).
A significant decrease in carbon contents of sample heated to 1040°C in 30 min is owing to either removing as CO and/or CO2 via the reduction reaction which causes a greater weight loss in this sample, or consuming via the Boudouard reaction according to Eq. (7).
| (7) |
No significant weight loss after a further heating to 1290°C demonstrates the accomplishment of reduction reaction at ca. 1040°C.
Figure 6 shows the CO/CO2 of the off-gas during microwave heating of sample heated to 1290°C in 35 min.
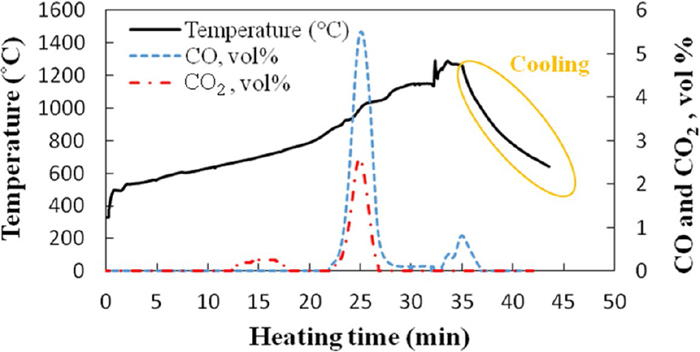
Off-gas analysis of sample heated to 1290°C in 35 min during microwave irradiation. (Online version in color.)
Simultaneous dehydration of Ca(OH)2 and binder removal at temperatures lower than 645°C cause a significant decrease in vol.% of CO2 in the off-gas. Therefore, no CO/CO2 was detected in the off-gas at temperatures lower than 645°C. On the other words, the presence of H2O in the off-gas (resulted from dehydration reaction) up to 645°C leads to a significant decrease in volume fraction of CO/CO2 while at temperatures >645°C, the H2O has no effect on the off-gas measurements owing to the fully dehydration up to 645°C. After binder removal and fully dehydration of sample at 645°C, see Fig. 3(d), an increase in the CO2 of the off-gas (ca. 0.3 vol.% CO2) followed by decreasing to zero vol.% at ca. 680°C was detected. Although no calcium carbonate peak was detected in the XRD pattern of sample after calcinations or after 2 min treatment, see Fig. 3(e), such CO2 generation is likely attributed to the calcination of possibly remaining CaCO3. Therefore, the weight loss at this temperature seems to be owing to the calcination of the small amount of calcium carbonate.
A significant increase in the CO and CO2 of the off-gas at ca. 850–1040°C is attributed to the onset of reduction reaction, Eqs. (2) and/or (3), at ca. 850°C after a treatment for 22 min. This result can be supported by detecting Fe phase in the XRD pattern of sample heated to 920°C, as shown in Fig. 3(c). Rosenqvist24) also reported that a temperature over 800°C is required for reduction of FeS–CaO–C mixture owing to the low reactivity of carbon at lower temperatures.
Furthermore, the absence of CO2 and a dramatic decrease in CO of the off-gas at temperatures over ca. 1040°C, not only, confirm that the reduction reaction is accomplished at 1040°C, which causes no significant weight loss at temperatures over 1040°C, but also, indicate that a greater carbon loss during treatment at temperatures over 1040°C, as shown in Fig. 5, is attributed to the carbon gasification via the Boudouard reaction, Eq. (7). In addition, stoichiometric calculations showed that ca. 9.1 mass% carbon remains after reaction whereas the measured carbon contents of samples heated to 1040 and 1290°C are 9 and 7.4 mass%, respectively. These calculations also confirm carbon consumption via carbon gasification reaction. On the other hand, XRD internal quantitative analysis, shown in Fig. 7, demonstrated a similar ratio of ICaS/ISi (Fig. 7(a)) at temperatures over 1040°C, confirming the accomplishment of total reaction, Eq. (4), at ca. 1040°C.
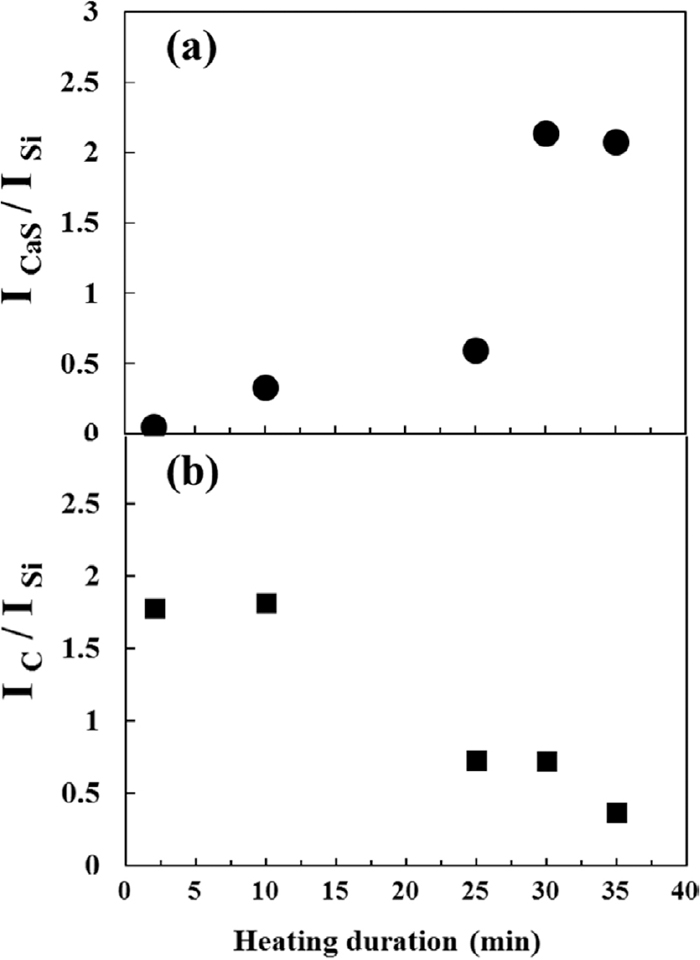
(a) ICaS/ISi and (b) IC/ISi of samples heated to certain temperatures. ICaS/ISi and IC/ISi were measured according to the XRD internal quantitative analysis by adding 10 mass % Si as standard material to samples.
On the other hand, oxygen balance can be calculated according to off-gas analysis. The input oxygen of 0.018 mol was estimated from initial CaO in the sample. The output oxygen of 0.011 mol was calculated from the measured CO/CO2 in the off-gas. These calculations also indicate oxygen removal during treatment. The difference between input and output oxygen is likely attributed to the presence of CaO in the sample heated to 1295°C in 35 min, see Fig. 3(a).
Moreover, it is reported that the reduced iron can absorb carbon at temperatures higher than 1200°C to make molten iron particles during microwave heating.22) Formation of molten iron particles was confirmed in sample heated to 1290°C by comparing the optical microscope and SEM-EDX images of samples heated to 1290 and 1040°C, shown in Figs. 8(a) and 8(b), respectively. Such behavior was also reported by Hara et al.22) who observed molten iron droplets during microwave heating of a magnetite and graphite mixture at temperatures higher than 1200°C.
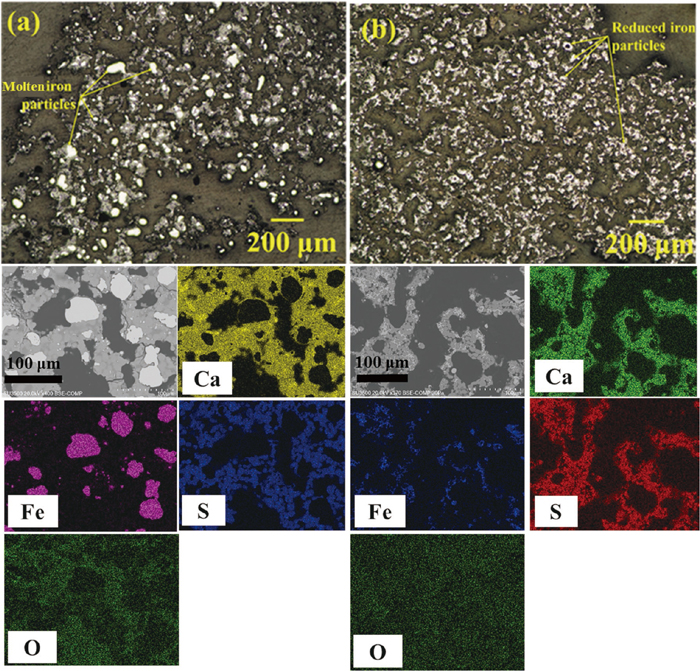
Optical microscope and SEM-EDX images of samples heated to (a) 1290°C in 35 min, and (b) 1040°C in 30 min via microwave irradiation.
Considering Fig. 8, Fe particles are distributed in a matrix of CaS. This indicates that iron oxide (product of the ion exchange reaction) was also distributed in the CaS matrix before reduction to metallic iron. Therefore, the absence of iron oxide phase despite detection of CaS in the XRD pattern of samples heated up to 645°C (see Figs. 3(e) and 3(d)) is likely attributed to a higher weight% of CaS (matrix) compared to the iron oxide particles in the XRD sample. Moreover, after a further progress in the ion exchange reaction at 920°C, FeO particles cannot be enormously detected owing to the formation of an oxysulfide phase (FeO∙CaS) which was detected by both XRD analysis (see Fig. 3(c)) and SEM-EDX observations (Fig. 4(b)).
3.2. Effect of Microwave Heating on Reduction DegreeReduction degree (%) was calculated according to Eq. (8):
| (8) |
Figure 9 shows the reduction degree of the FeS:CaO:C = 1:1:2 mixture during microwave heating of samples heated to 1290°C and that of a mixture with a same mole ratio after conventional heating reported by Jha et al.1) and by Kutsovskaya et al.20)
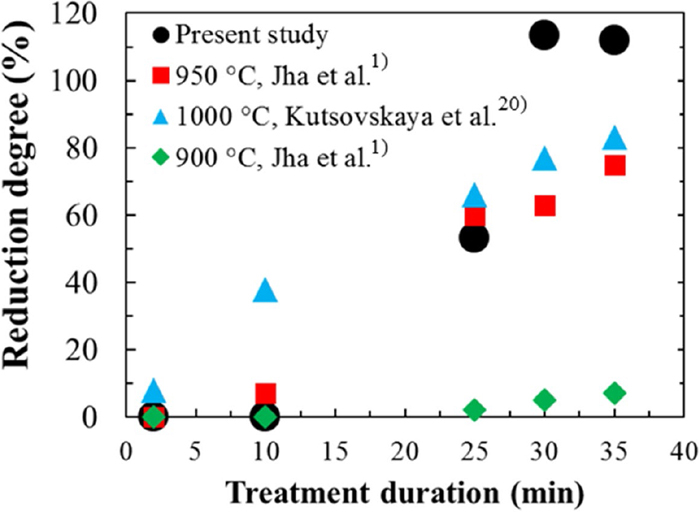
Reduction degree of FeS–CaO–C mixture during microwave heating to a certain temperature (circle), after conventional heating at 950°C for ca. 35 min reported by Jha et al.1) (square), after conventional heating at 1000°C for ca. 35 min reported by Kutsovskaya et al.20) (triangle), and after conventional heating at 900°C for ca. 35 min reported by Jha et al.1) (rhombus). (Online version in color.)
It was observed that the reduction degree of samples heated for 30 and 35 min is higher than 100%. Jha et al.1) reported also reduction degree over 100% and theorized that it is because of the drainage of liquid form pellet. In this study, the other possibility is reduction of FeO via Eqs. (3) and/or (9) followed by carbon gasification via the Boudouard reaction according to Eq. (7) which causes a larger weight loss than the stoichiometric weight change. A decrease in carbon contents of sample heated to 1290°C, despite the accomplishment of the reduction reaction at 1040°C, confirms the carbon consumption via the Boudouard reaction which can be promoted by microwave irradiation.25)
| (9) |
Jha et al.1) showed that a minimum 90 min is required to initiate the reduction reaction in a FeS:CaO:C = 1:1:2 mixture at 850°C during conventional heating whereas Ishizaki et al.26) reported a rapid reduction of FeO to Fe at 870–1200°C during microwave heating of a magnetite-coal composite pellet. In the present study, as mentioned above, the reduction reaction was initiated at ca. 850°C after a 20 min microwave heating. It should be mentioned that conventional heating of sample has been conducted at a constant temperature in the literature. It means the sample needs to be heated for certain duration at a specific temperature whereas during microwave heating in the present study, sample properties were studied as a certain temperature was attained, without any holding time. This indicates extraordinary effect of microwave heating on the reaction progress.
This result is in a good agreement with previous investigations9,15) wherein the carbothermic reduction of iron oxide is conducted in a shorter time and at a lower temperature during microwave heating than the conventional heating. Further, Kashimura et al.11) reported an enhancement in iron production from magnetite and graphite during microwave heating. Also, Hunt et al.25) reported a significant decrease in apparent activation energy of the Boudouard reaction during microwave irradiation compared to conventional heating. Therefore, the improvement in reaction progress in the present study would be attributed to the effect of microwave irradiation on the chemical reactions.11,25,27,28)
Carbothermic reduction behavior of FeS:CaO:C = 1:1:2 mixture during microwave heating was investigated using a multi-mode microwave generator at 1050 W at 2.45 GHz. Results can be summarized as follows:
(1) FeO consumes via reduction reaction that causes a significant progress in the desulfurization reaction.
(2) According to off-gas analysis, reduction reaction initiates at ca 850°C after microwave heating for ca. 20 min that is shorter than the time required for the carbothermic reduction of FeO to Fe at 850°C during conventional heating.
(3) Reduced iron can absorb carbon during microwave heating at temperatures higher than 1200°C to make molten iron particles.