2019 年 59 巻 7 号 p. 1198-1204
2019 年 59 巻 7 号 p. 1198-1204
To reduce CO2 emissions from steel works, low reducing agent rate (low coke rate) operation of the blast furnace is desired. Mixing nut coke in the ore layer is one effective measure for realizing this type of operation. Therefore, the effect of coke mixing on the reduction reaction rate of ore and the gasification reaction rate of coke in the mixed layer of ore and coke was investigated. The reduction rate of the ore and the gasification rate of the coke in the mixed layer of ore and coke was estimated by a reduction and gasification experiment, and the packing structure of the coke in the mixed layer was estimated by a mathematical model analysis using the discrete element method. The reduction rate of the ore and the gasification rate of the coke in the mixed layer was affected by the degree of contact between the ore and coke. In addition, the reduction rate of the ore and the gasification rate of the coke in the mixed layer was accelerated by the effects of mutual utilization of the gases generated by the reactions.
In order to reduce environmental problems such as global warming, reduction of CO2 emissions is desired, and low reducing agent rate (low coke rate) operation of the blast furnace is expected at integrated steel works. In order to achieve low reducing agent rate operation, burden distribution control techniques such as control of the ore to coke layer thickness in the radial direction have been proposed and implemented. Recently, it has also become necessary to improve permeability in response to deterioration of the grade of raw materials. Therefore, a coke and ore mixed charging technology which is compatible with improvement of reduction efficiency and permeability has been expected, and this type of technology has been implemented in many blast furnaces.1,2,3,4,5,6)
When coke is mixed in the ore layer in a blast furnace, the reduction reaction of the ore and the gasification reaction of the carbonaceous material are simultaneously accelerated.7) This is called a coupling reaction. When the ore and carbonaceous material are arranged in close proximity, it is assumed to be for mutual utilization of the local CO and CO2 gases generated by the reduction and gasification reactions, and a local temperature increase in the carbonaceous material by the heat of the reduction reaction. Some fundamental experiments have been carried out to clarify these phenomena.8)
Studies on the effects of coke and ore mixed charging on the reduction and gasification reactions have also been conducted, and the influence of coke reactivity on reduction behavior has been investigated.9,10,11) The effects of the mixed coke rate and mixed coke particle size on reduction behavior and permeability have also been investigated.5,6,12,13,14,15) However, in many of these studies, the phenomena were confirmed experimentally, but there are few examples of systematical investigation. Sunahara et al.11) and Nouchi et al.16,17) conducted reduction and gasification experiments in which particles ranging from the millimeter order to the micron order were mixed. The average interparticle distance of the ore and carbonaceous material was defined, and the fact that the gasification starting temperature of the carbonaceous material decreased as the average interparticle distance became smaller was confirmed. In case of mixing coke in ore layer, possibility of the decrease in the gasification starting temperature by close arrangement of the ore and carbonaceous material was indicated. Sunahara et al.11) was performed the analysis for the reaction rate of the reduction and gasification to simulate the experimental results. And the necessary of the mathematical model considering about the influence of close arrangement of the ore and carbonaceous material was indicated to simulate the experimental results because the effect of the ore and the coke arrangement in the height direction in the crucible on calculation results was small. However, it was not investigated about the mathematical model to simulate the phenomena. Ueda et al.18) proposed the composed reaction model considering about local reaction between the reduction of iron ore and the gasification of coke through the gas phase in the vacancy, and simulated the reaction behavior of the carbon iron ore composite. Thus, the reaction model considering about local reaction behavior is efficient to simulate the effect of the close arrangement of the ore and carbonaceous material. As a further detailed simulation study, several numerical simulations on the effect of the particle arrangement of carbonaceous materials and ores on the reduction and gasification reactions in the ore layer have been conducted.19,20) However, since these studies focused on the vertical arrangement of the particle layers in the packed bed, the effect of the individual particle arrangements on the reduction and gasification reactions and the close arrangement of the ore and carbonaceous material was not discussed.
In this study, first, an isothermal reduction experiment was conducted to investigate the reduction behavior of ore and the gasification behavior of coke in a mixed layer of ore and coke, and the effects of the mixed coke rate and coke particle size on the reduction and gasification reactions were evaluated. Next, in order to investigate the effect of the ore and coke particle arrangement in the mixed layer of ore and coke on the reduction behavior of the ore and the gasification behavior of the coke, a mixed packed bed of ore and coke was calculated by the discrete element method (DEM), and the mixing condition of the coke in the mixed layer was evaluated quantitatively. Finally, a reduction and gasification reaction model considering the arrangement of the ore and coke particles was constructed, and the effect of the mixing condition of the coke in the mixed layer on the behavior of the reduction and gasification reactions was evaluated.
The effect of coke mixing on the reduction behavior of sinter was investigated experimentally. A schematic diagram of the experimental apparatus is shown in Fig. 1. The properties of the sinter and coke used in the experiments are shown in Table 1. The sinter and coke were crushed and sized, and the resulting sample materials were used in this experiment. When the sinter and coke were mixed, a predetermined amount of coke having predetermined particle diameter was mixed homogeneously in a sinter bed of 500 g sinter with a size of 15–20 mm. To examine the effect of the mixed coke ratio, the mixed coke ratio was changed in the range between 1.9 and 11.3 mass%, while its particle diameter was kept in the same range of 15–20 mm. On the other hand, to examine the effect of the particle diameter of mixed coke, the diameter was changed from 10 to 24 mm under the constant mixed ratio of 3.8 mass%.

Schematic diagram of experimental apparatus. (Online version in color.)
| T–Fe | FeO | SiO2 | Al2O3 | CaO | MgO | |
|---|---|---|---|---|---|---|
| Sinter | 56.6 | 8.95 | 5.36 | 1.52 | 11.1 | 1.20 |
| Ash | V.M. | F.C. | S | SiO2 | Al2O3 | |
|---|---|---|---|---|---|---|
| Coke | 11.9 | 0.8 | 87.3 | 0.47 | 6.28 | 3.33 |
The coke was homogeneously mixed in the sinter bed, and the mixture is put into the reaction tube (inner diameter: 75 mm). The mixed bed was then heated to the experimental temperature (1000°C) under flowing N2 gas from the bottom of the reaction tube. After reaching the experimental temperature, the sample was held for 30 min, after which the flowing gas was switched to CO/N2 (= 30/70). After reaction for 3 h at a constant temperature, the gas was switched to N2 and the temperature was lowered. The gas flow rate was 15 NL/min during the whole period of the experiment. After cooling to room temperature, the sample was removed, and the sinter and coke were separated and weighed. The reduction degree of the sinter was calculated as the ratio of the measured weight loss of the sinter to the mass of oxygen of iron oxide contained in the original sinter sample.
The effect of the mixed coke ratio on the reduction degree of the sinter and the effect of the mixed coke ratio on the weight loss of the coke are shown in Figs. 2 and 3, respectively. As the mixed coke ratio increased, the reduction degree of the sinter increased. This is due to the effect of mutual utilization of the gases generated by the reactions. It is considered that CO is regenerated by the gasification reaction with the mixed coke and the CO2 generated by the reduction reaction, and the regenerated CO then acts as a reducing agent in the reduction reaction of the sinter. As a result, the reduction rate of the sinter increased.

Effect of mixed coke ratio on reduction degree of sinter. (Online version in color.)

Effect of mixed coke ratio on weight loss of mixed coke. (Online version in color.)
The weight loss of the coke was increased by the increase in the mixed coke ratio. However, the weight loss of the coke per coke particle was decreased slightly by the increase in the mixed coke ratio. When the mixed coke ratio is high, it is considered that the CO2 generated by the reduction reaction reacts with the mixed coke as the gasification reaction, but the reaction efficiency per coke particle is low.
Next, the effects of the mixed coke particle diameter on the reduction degree of the sinter and the weight loss of the coke are shown in Figs. 4 and 5, respectively. As the mixed coke particle diameter decreased, the reduction degree of the sinter and the weight loss of the coke increased. As the particle diameter of the mixed coke decreased, the specific surface area of the mixed coke increased and the gasification reaction rate of the mixed coke also increased. Therefore, it is considered that the reduction reaction rate increased because the amount of CO generated by the gasification reaction between the CO2 generated by the reduction reaction and the mixed coke increased. The weight loss of the coke per coke particle decreased as the mixed coke particle diameter became smaller. When the mixed coke particle diameter is small, the reaction efficiency per coke particle is low, as in the case of a high mixed coke ratio.

Effect of mixed coke diameter on reduction degree of sinter. (Online version in color.)

Effect of mixed coke diameter on weight loss of mixed coke. (Online version in color.)
The experimental results were compared with the calculated values using the conventional equation of the overall reaction rate,21) and the effect of coke and sinter mixed charging on the reaction rate considering the mutual utilization of gases generated by the reduction and gasification reactions was estimated. Equation (1) was used for the overall reaction rate of the reduction reaction (R1).
| (1) |
| (2) |
In this model, the gasification reaction rate was calculated by using the concentration of CO2 that was generated in the reduction reaction in the layer, while the reduction reaction rate was calculated by using the concentration of CO that includes CO generated by the gasification reaction and supplied CO. Thus, the model takes into account the effects of the mutual utilization of the gases generated by the reactions. Sunahara et al. reported that the effect of the longitudinal distribution of gas composition in the crucible on the calculation results was small.11) Thus, the model assumed uniform distributions of the temperature and the gas composition in the crucible.
Here, the ratios of the experimentally obtained overall reaction rates to the ones calculated by the reaction rate equations were defined as α1 and α2. The acceleration coefficients α1 and α2 were for the reduction reaction and for the gasification reaction, respectively. Under the non-mixing condition, the values of both α1 and α2 were 1.0. Note that the reduction and gasification rates under the non-mixing condition were given by the values multiplying 0.77 and 1.30 on Eqs. (1) and (2), respectively, according to the preliminary experimental results.
The effect of the mixed coke ratio on α1 and α2 is shown in Fig. 6. Here, α1 was approximately 1.0 regardless of the mixed coke ratio, whereas α2 was higher than 1.0 regardless of the mixed coke ratio. Next, the effect of the coke particle diameter on α1 and α2 is shown in Fig. 7. The value α1 was approximately 1.0 regardless of the mixed coke particle diameter. However, α2 was higher than 1.0 regardless of the mixed coke particle diameter.

Effect of mixed coke ratio on reaction rate parameter. (Online version in color.)
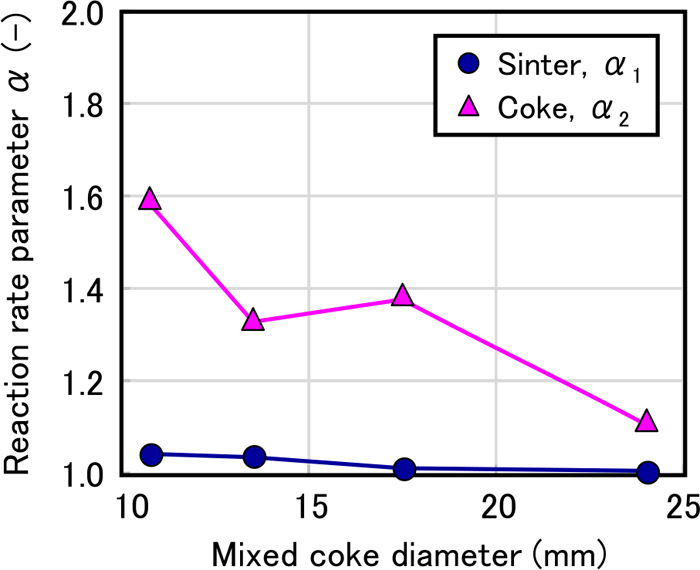
Effect of mixed coke diameter on reaction rate parameter. (Online version in color.)
Therefore, it was estimated that the acceleration of the local gasification reaction of coke had a great effect as an effect of coke mixing in the sinter bed on the reduction reaction of the sinter.
4.2. Particle Arrangement of Coke in Mixed Packed Bed of Sinter and CokeIn the previous research, it was reported that the reduction reaction and the gasification reaction are accelerated by a close arrangement of iron and carbonaceous materials.8) In the case of a mixed packed bed of sinter particles and coke particles, the iron and carbonaceous materials are arranged in close proximity in the vicinity of the contact points between the sinter particles and coke particles. Based on this, it is assumed that the contact point between sinter and coke affects the acceleration of the local coke gasification reaction. Therefore, the condition of contact between the sinter particles and coke particles in the mixed packed bed of sinter and coke was investigated.
The mixing behavior of the sinter particles and coke particles was simulated by the Discrete Element Method (DEM). PFC3D, a three-dimensional particle behavior analysis software, was used for this analysis. In DEM, translational and rotational movements change due to the stress and moment acting between individual particles. The contact force was divided into the normal direction and the tangential direction and calculated by the Voigt model represented by the spring and dashpot shown in Fig. 8. In order to consider the interaction of the frictional effect, a slider was considered in the shear direction. The packing behavior of the mixed particles of sinter and coke was calculated under conditions simulating the experiment in Section 2. In this calculation, the sinter particle and coke particle were assumed to be spherical. Sinter particles and coke particles were randomly generated in a cylindrical vessel (inner diameter: 75 mm) and filled, and the contact condition between the sinter and coke was evaluated. Each condition was calculated 30 times, and the average value was evaluated as the number of contacts.

Schematic diagram of discrete element model. (Online version in color.)
Figure 9 shows the calculation results of a typical packed bed of sinter and coke under each condition. The coke particle number was increased by an increase in the mixed coke ratio or a decrease in the mixed coke particle diameter. Under all conditions, it was observed that the coke was dispersed and mixed in the packed bed.

Calculation result of mixing conditions of sinter and coke by DEM. (Online version in color.)
Figure 10 shows the effect of the mixed coke ratio on the number of the sinter-coke contact points per sinter particle in the mixed packed bed (here-in-after, N1). The contact number N1 was increased by an increase in the mixed coke ratio. Figure 11 shows the effect of the mixed coke ratio on the number of contact points between the sinter and coke per coke particle in the mixed packed bed (here-in-after, N2). An increase in the mixed coke ratio decreased N2. These results agree qualitatively with the changes in the reduction degree of the sinter and the weight loss of the coke per coke particle in Figs. 2 and 3.

Effect of mixed coke ratio on contact number between coke and sinter per sinter particle. (Online version in color.)

Effect of mixed coke ratio on contact number between coke and sinter per mixed coke particle. (Online version in color.)
Next, Fig. 12 shows the effect of the mixed coke particle diameter on N1. The value of N1 was decreased by an increase in the mixed coke particle diameter. Figure 13 shows the effect of the mixed coke particle diameter on N2. An increase in the mixed coke particle diameter increased N2. These results agree qualitatively with the change in the reduction degree of the sinter and the weight loss of the coke per coke particle in Figs. 4 and 5.

Effect of mixed coke diameter on contact number between coke and sinter per sinter particle. (Online version in color.)

Effect of mixed coke diameter on contact number between coke and sinter per mixed coke particle. (Online version in color.)
Therefore, when the sinter and coke were mixed and filled, the reduction reaction rate and gasification reaction rate were accelerated by the coupling reaction in the vicinity of the contact points between the closely-arranged sinter particles and coke particles, and it is assumed that the reduction degree of the sinter and the weight loss of the coke in the packed bed increased as a result of the accelerated reactions.
4.3. Mathematical Model Analysis of Reduction Raction and Gasification ReactionIt was assumed that the reduction reaction rate and the gasification reaction rate were accelerated by the coupling reaction in the vicinity of the sinter-coke contact points. For example, when iron and carbonaceous materials are arranged close to each other at a distance smaller than the millimeter size, as in the case of ferro-coke, it has been reported that the starting temperature of the gasification reaction decreases by about 150°C.16,22,23) Here, a mathematical model analysis of the reduction reaction rate and gasification reaction rate was carried out assuming that the starting temperature of the gasification reaction in the vicinity of the sinter-coke contact point decreased 150°C, as in the above-mentioned literature.
The analysis of the reduction reaction and gasification reaction was conducted by the same method as in Section 4.1. Here, the reduction reaction rate and gasification reaction rate in the vicinity area of the sinter-coke contact point and in the other area were separately calculated. The surface areas of the particles in the vicinity of the sinter-coke contact point and the surface areas of the particles in the other area were calculated. The average reduction reaction rate and average gasification reaction rate in the packed bed were shown in Eqs. (3) and (4), and the reduction reaction rate of the sinter and gasification reaction rate of the coke in the mixed packed bed of sinter and coke were calculated.
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |

Schematic diagram of influence area by closely arrangement effect between sinter and mixed coke. (Online version in color.)
A reduction and gasification reaction rate analysis was carried out under the same conditions as the reduction and gasification experiment in Section 2 using Eqs. (1), (2), (3), (4), (5), (6), (7), (8), (9). Figure 15 shows the comparison of the calculated and experimental results of the reduction degree of sinter, and Fig. 16 shows the comparison of calculated and experimental results of the carbon gasification ratio of coke per coke particle. Both calculation results were in good agreement with the experimental results.

Comparison of calculated reduction degree and reduction degree obtained by experiment. (Online version in color.)

Comparison of calculated carbon gasification ratio and carbon gasification ratio obtained by experiment. (Online version in color.)
Therefore, it was considered that the local gasification reaction was accelerated by the coupling reaction in the vicinity of the sinter-coke contact points in the mixed packed bed of the sinter and the coke. The average reduction reaction rate and average gasification reaction rate in the packed bed were accelerated by mutual utilization of the gases generated by the reduction reaction and gasification reaction in the vicinity of the sinter-coke contact points.
The effects of the mixing condition of sinter and coke on the reduction reaction of the sinter and the gasification reaction of the coke in a mixed packed bed of sinter and coke were investigated by an experiment and mathematical model analysis. The following results were obtained.
(1) The reduction reaction rate of sinter and the gasification reaction rate of coke were increased by mixing coke in the sinter layer, and this was due to the acceleration of the local gasification reaction of the coke.
(2) When sinter and coke were mixed, the reduction reaction rate and gasification reaction rate were accelerated in the vicinity of the sinter-coke contact points arranged in close proximity to each other.
(3) According to the mathematical model analysis, the acceleration of the local gasification reaction rate in the vicinity of the sinter-coke contact points accelerates the average reduction reaction rate and average gasification reaction rate in the packed bed by mutual utilization of the gases generated by the reduction reaction and gasification reaction in the vicinity of the sinter-coke contact points.