2019 Volume 59 Issue 8 Pages 1376-1381
2019 Volume 59 Issue 8 Pages 1376-1381
Chemical structure of coal is evolutionary changed during pyrolysis that accompanies gas release. The chemical structural change and gas formation profiles play important roles in determining caking property and physical properties such as strength and size of the resultant coke. However, analyses of volatile components and structural analysis of solid char have been mostly performed individually, and it is difficult to combine both and to obtain quantitative understanding on the thermal decomposition of coal at mechanistic level. In this study, simultaneous analyses of solid chemical structures of the heat treated coals and gas formation profiles were conducted for two kinds of coals that were pyrolyzed at an identical condition. On-line gas analysis with a quadrupole mass spectrometer and spectroscopic methods (NMR and FT-IR) were employed for quantitative evaluation of gas formation characteristics and solid chemical structure, respectively. The information obtained were then integrated to acquire new insight for coal pyrolysis mechanism. Here an approach to quantify the transferable hydrogen that contributes to stabilize radicals formed in pyrolyzing coal was proposed. It includes the quantitative assessment of aromatic cluster growth, decomposition of hydroxyls, and releases of hydrogen and pyrolytic water into gas phase. The proposed approach suggested that a bituminous coal that exhibits plasticity during pyrolysis had 3.5 mol/kg-coal transferable hydrogen, whereas the amount of transferable hydrogen of the sub-bituminous coal, a non-caking coal, was 1.3 mol/kg-coal, during pyrolysis up to 500°C.
Changes in the chemical structure of coal during the dry distillation process and the formation characteristics of volatile components are important factors related to the mechanism for exhibiting caking property of coal as well as the physical properties of the resultant coke lump. Therefore, these were analyzed and studied by various methods.1,2,3,4,5,6,7,8,9) However, in the past, analysis of volatile components and chemical structure of the solid char were mostly performed individually, and it is difficult to combine both and to obtain quantitative understanding on the thermal decomposition reaction mechanism.
In understanding the caking property of coal, the occurrence of the transferable hydrogen was suggested.10,11,12) The transferable hydrogen is formed within solid and/or liquid phase of coal undergoing thermal decomposition. The existence of the transferable hydrogen is not directly observed at all. However, the transferable hydrogen is believed to be a key species for stabilizing radicals formed by thermolysis by preventing the crosslinking reactions, and contributes to enhance softening/melting, that is a required properties of coal for producing lump coke used in a blast furnace.
Several methods were proposed to detect and estimate the amount of the transferable hydrogen. Sanada et al.10) studied the amount of the transferable hydrogen in coal by quantifying the amount of hydroanthracene produced after the heat treatment of the mixture of the coal and anthracene. Similar method using polycyclic aromatic hydrocarbons to dehydrogenate coal was also reported.2,5,11) Larsen et al.12) used sulfur or quinones for abstracting hydrogen of coal. However, it is evident that the all methods to quantify transferable hydrogen by heating the mixture of coal and the hydrogen accepters such as polycyclic aromatic hydrocarbons and sulfur almost always suffer from the problems of accessibility of the hydrogen accepters into the coal macromolecular network and side reactions.
This paper reports a new approach in quantifying transferable hydrogen without using any ligand for coal dehydrogenation. The approach includes quantitative studies of size growth of aromatic clusters occurred in coal char upon heating revealed by the combination of solid state high resolution 1H- and 13C-NMR spectroscopies, decomposition of hydroxyls revealed by the FT-IR, and release of hydrogen and pyrolytic water into gasphase measured by on-line gas analysis with a quadrupole mass spectrometer. This made the quantification of the transferable hydrogen possible.
The aromatic cluster size is increased with increasing the pyrolysis temperature. The growth of aromatic cluster means condensation reaction of aromatic ring systems in coal chemical structure and must accompany hydrogen formation. We focus on the fate of the hydrogen simultaneously formed by the aromatic cluster growth. It can be roughly classified into two groups; hydrogen released to gasphase and hydrogen consumed by reactions in solid/liquid phase. The latter can further be divided into hydrogen used for the formation of pyrolytic water and the transferrable hydrogen that contribute for quenching the radicals formed during coal pyrolysis. Two types of coals including sub-bituminous and bituminous coals were studied to highlight the difference in their caking properties based on the estimated amounts of the transferable hydrogen.
A bituminous coal (NG) and a sub-bituminous coal (AD) were employed as the starting coal samples. The ultimate analyses are listed in Table 1. The coal samples were pulverized to less than 75 μm and dried under vacuum for 24 h. Amounts of hydroxyl groups in NG and AD were estimated to be 1.6 and 5.4 mol-OH/kg-coal, respectively, based on the correlation established by Yarzab et al.13)
| Coal | NG (bituminous) | AD (sub-bituminous) |
|---|---|---|
| Elemental composition, wt% on dry basis | ||
| C | 80.8 | 70.0 |
| H | 4.6 | 5.2 |
| N | 1.8 | 0.9 |
| O+S (by diff.) | 5.0 | 23.4 |
| ash | 7.8 | 0.5 |
A 2.0 g of the coal sample located at the center of a horizontal quartz tubular reactor was heated up to 900°C with a heating rate of 5°C/min in, using Ar as carrier gas with a constant flow rate of 200 ml/min (STP). After passing through quartz wool at the tail end of the reactor, the volatile products from the pyrolysis were detected by a quadrupole mass spectrometer (Q-MS, M-QA 200TS, ANELVA Corp.). The volatile products were introduced into the Q-MS through a capillary, which was convolved by tape heater of 200°C to avoid condensation of water included in the volatiles. A diaphragm pump was used to help transporting the gas into the Q-MS by reducing the tube pressure to 25 mbar. The vacuum degree of Q-MS was kept at 1×10−5 Pa before measurement, and at 1×10−3 Pa during measurement. Other details of Q-MS operation were set as follows: ionizing voltage, 24 eV; emission current, 1.0 mA; mass number range, 1–80 m/z; scan speed, 100 ms/amu. Mass numbers of 2, 15, 18, 28, and 44 were selected to characterize the presences of H2, CH4, H2O, CO, and CO2, respectively. The detailed protocols for the gas analysis are provided in elsewhere.9)
2.3. Heat TreatmentA 1.0 g of NG or AD was heated up to different target temperatures ranging from 400 to 900°C at a heating rate of 5°C/min with a N2 flowrate of 200 ml/min (STP) in a vertical quartz tube reactor. Once reaching the target temperatures, the chars were instantaneously cooled down by falling into the bottom of the reactor cooled externally by liquid N2. Masses and elementary compositions of the heat treated samples (chars) were measured and analyzed, respectively. The mass changes upon the heat treatments were almost identical with those measured continuously by thermogravimetry. The chars were also studied by solid-state 13C/1H-NMR and FT-IR.
2.4. Spectroscopic Analyses of Coal and Heat Treated Coal Samples 2.4.1. 13C and 1H-NMRThe 13C- and 1H-NMRspectra were recorded with the DEPTH2 and wPMLG techniques, respectively, at JEOL ECA 400 spectrometer (400 MHz 1H and 100.53 MHz 13C frequencies). The repetition time was 20 s. Magic angle spinning was performed at 15 kHz in the commercial probe (JEOL 4 mm CPMAS). The 13C NMR data were then analyzed by a variation of the method described by Solum,14) whereas the 1H NMR spectra were analyzed by decomposing into two peaks arising from aromatic and aliphatic hydrogen.
2.4.2. FT-IRThe FT-IR spectra of the coal samples and cokes were recorded on a FT-IR spectrometer (PerkinElmer Spectrum Two) with universal attenuated total reflectance (UATR) accessories. The UATR employs a DiCompTM crystal, which is composed of a diamond ATR with a ZnCe focusing element. The spectrum acquisition was conducted in the range from 4000 to 450 cm−1 at a resolution of 1 cm−1.
The 13C-NMR spectra for the starting coal samples (NG and AD) are presented in Fig. 1. According to the previous studies,14,15) the carbon in the coal and heat treated samples were classified into 8 different types, which are methyl (-CH3), methylene (-CH2), aliphatic carbon with oxygen substituents (Al–O), protonated aromatic carbon (Ar–H), aromatic carbon with alkyl groups (Ar–C), bridgehead carbon, aromatic carbon with oxygen substituents (Ar–O), and carbonyl carbon (O=C–O, C=O). The amount of each carbon were evaluated based on the areas of resolved peaks by deconvoluting the spectra. The peak assignments are also given in Fig. 1. Figure 2 displays the 1H-NMR spectra for the starting coal samples (NG and AD), which are resolved into aliphatic and aromatic hydrogen. The amounts of aliphatic and aromatic hydrogens for the coal and heat treated samples also evaluated by decomposing the spectra as shown in Fig. 2.

13C-NMR spectra for bituminous coal NG (a) and sub-bituminous coal AD (b) and the deconvoluted peaks with assignments of different carbon types. (Online version in color.)

1H-NMR spectra for bituminous coal NG (a) and sub-bituminous coal AD (b) and the deconvoluted peaks with assignments of different hydrogen types. (Online version in color.)
The FT-IR spectra for the starting coal samples (NG and AD) are presented in Fig. 3. The spectra were deconvoluted to estimate the amounts of hydroxyls for the coal and heat treated samples. An established method16,17) was used for peak decomposition and assignments. The amounts of the hydroxyls remained in the heat treated samples were estimated based on the Eq. (1).
| (1) |

FT-IR spectra for bituminous coal NG (a) and sub-bituminous coal AD (b) and the deconvoluted peaks with assignments of different bands. (Online version in color.)
The definitions of the abbreviations used in the Eq. (1) are listed in Table 2.
| Abbreviation | unit | Definition |
|---|---|---|
| COH, T | mol/kg-coal | Amount of hydroxyls remained for heat treated coal samples at temperature T |
| COH, original | mol/kg-coal | Amount of hydroxyls in the original coal samples 1.6 and 5.4 for NG and AD, respectively |
| AO–H, T | Arbitrary | Total area for FT-IR peaks assigned to hydroxyl OH stretching vibrations for heat treated coal samples at temperature T |
| AAromaC–H, T | Arbitrary | Total area for FT-IR peaks assigned to aromatic CH stretching vibrations for heat treated coal samples at temperature T |
| AO–H, original | Arbitrary | Total area for FT-IR peaks assigned to hydroxyl OH stretching vibrations for the original coal samples |
| AAromaC–H, original | Arbitrary | Total area for FT-IR peaks assigned to aromatic CH stretching vibrations for the original coal samples |
| Caroma, T | mol/kg-coal | Amount of aromatic carbons for heat treated coal samples at temperature T |
| Caroma, original | mol/kg-coal | Amount of aromatic carbons in the original coal samples |
Figure 4 shows the variation of the release rates of hydrogen and water from the coal samples under heating as a function of temperature. The rates of water formation for both sample give two peaks. The peak at around 100°C corresponds to the release of water physically absorbed in the samples, whereas the peak at around 450–500°C corresponds to the release of water formed by the decomposition of oxygen containing functional groups in coal samples due to pyrolysis. The temperature at which water evolution rate exhibits maximum is at around 140°C for AD, and higher than that for NG. AD possesses higher amounts of oxygen functional groups that hold water via hydrogen bonds.18,19) The strongly sorbed water mainly contributes the higher temperature for the water release upon heating than the boiling point of pure water. Based on this measurement, it is possible to quantify the hydrogen and pyrolytic water generated by pyrolysis.

Formation rate profiles of hydrogen and water for bituminous coal NG (a) and sub-bituminous coal AD (b) during heating with a rate of 5°C/min under atmospheric Ar flow as measured by on line gas analysis with a quadrupole mass spectrometer. (Online version in color.)
By combining the data obtained from 13C and 1H-NMR measurements, it is possible to evaluate mole fraction of bridgehead aromatic carbons in the total aromatic carbons, χb. χb corresponds to the degree of aromatization. Larger χb value means larger aromatic cluster size in coal. Figure 5 shows the χb values for NG and AD as a function of the heat treated temperature. The possible aromatic clusters are also indicated. The χb increases with increasing the pyrolysis temperature.

χb values for NG and AD versus the heat treated temperature. χb is mole fraction of bridgehead aromatic carbon and corresponds to the degree of aromatization. (Online version in color.)
Condensation reactions between aromatic ring systems can happen in coal pyrolysis and lead to the growth of aromatic clusters in coal macromolecular structure. Based on the stoichiometry, this reaction accompanies hydrogen formation as shown in Fig. 6. Here we estimated the possible amount of hydrogen evolved with heating of the coal samples up to 500°C, by using the information of the aromatic cluster structures of the starting NG and AD (at room temperature) and their chars obtained from the heat treatment at 500°C. The amount of aromatic carbon quantified by the 13C-NMR measurements provide molar amount of the aromatic cluster per unit mass of the coal samples and also provide absolute values for the amount of hydrogen possibly evolved due to the aromatic cluster growth. The possible amounts of hydrogen produced by heating of NG and AD up to 500°C are thus calculated to be 3.7 and 3.4 mol/kg-coal, respectively. The values correspond the bar graph numbered (0) indicated in Figs. 7(a) and 7(b) for NG and AD, respectively.
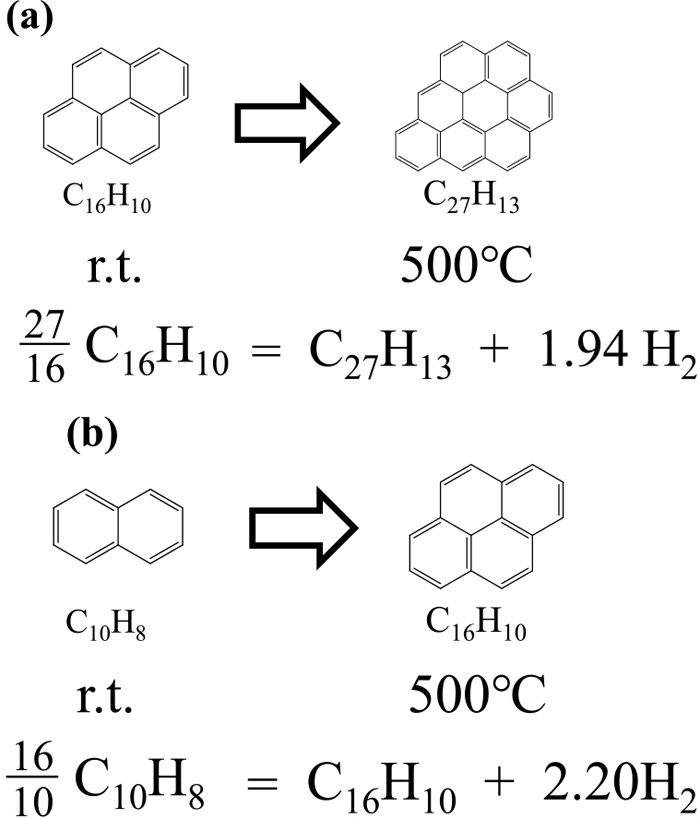
Possible hydrogen formation stoichiometry for NG (a) and AD (b) heated up to 500°C.

Amounts of hydrogen formed by the aromatic cluster growth and their fates upon heating up to 500°C for NG (a) and AD (b). (Online version in color.)
There are three possible fates for hydrogen generated by aromatic cluster growth as schematically drawn in Fig. 8. They include the hydrogens that are; (1) released to gas phase as molecular hydrogen, (2) consumed by hydro-deoxygenation reaction and converted to water, and (3) consumed to stabilize radicals formed by the thermal decomposition. The third hydrogen can be classified into the transferable hydrogen. The amounts of hydrogen released to gas phase as molecular hydrogen upon heating up to 500°C can easily be known based on the on-line gas analysis results as shown in Fig. 5, and are 0.06 and 0.08 mol/kg-coal for NG and AG, respectively. The values correspond the bar graph numbered (1) indicated in Figs. 7(a) and 7(b) for NG and AD, respectively.

Schematic drawing for conversion routes of hydrogen generated by aromatic cluster growth upon coal pyrolysis.
The amounts of pyrolytic water formed during pyrolysis up to 500°C were estimated to be 0.3 and 2.8 mol/kg-coal for NG and AD, respectively, by integrating the water formation rates in Fig. 4 from 300 to 500°C. The most possible mechanism for water formation is a condensation reaction by two hydroxyl groups. One mole of water can be produced from two moles of hydroxyls. It is thus possible to quantify the pyrolytic water formed through this mechanism using the values for the decreased amounts of hydroxyls upon heating. Based on the amount of the hydroxyls in the starting coals, 13C-NMR and FT-IR data for the starting coal and the heat treated samples, the amounts of the decomposed hydroxyls upon heating up to 500°C for NG and AD can be estimated to be 0.4 and 1.8 mol/kg-coal, respectively. The half of each value corresponds to the amount of pyrolytic water formed by the hydroxyls condensation reaction, and was calculated to be 0.2 and 0.9 mol/kg-coal for NG and AD, respectively. The values are lower than the amounts of pyrolytic water for both NG and AD, indicating that the hydroxyls condensation reaction is not enough to explain total amount of pyrolytic water. Another possible reaction that leads to pyrolytic water is hydro-deoxygenation. Hydrogen reacts with oxygen included in the coal chemical structure and converts into pyrolytic water. The amounts of hydrogen consumed for the dehydrogenation can correspond to the difference between the amounts of pyrolytic water released into gas phase and water generated by hydroxyls condensation reaction, and were calculated to be 0.1 and 1.9 mol/kg-coal for NG and AD, respectively. The values correspond the bar graph numbered (2) indicated in Figs. 7(a) and 7(b) for NG and AD, respectively. The hydro-deoxygenation reactions in AD are occurred more extensively than NG, because AD is the oxygen rich sub-bituminous coal.
3.4.4. Transferable HydrogenFinally, it is possible to estimate the amounts of transferable hydrogen as long as the three pathways including release into gas-phase, hydro-deoxygenation, and radical quenching (transferable hydrogen) can satisfactory explain the conversion routes for the hydrogen generated upon aromatic cluster growth. The difference between the amounts of the hydrogen generated upon aromatic cluster growth and the summation of the amounts of hydrogens released into gas-phase and used for hydro-deoxygenation. The amounts are estimated to be 3.5 and 1.3 mol/kg-coal for NG and AD, respectively. The values correspond to the bar graph numbered (3) indicated in Figs. 7(a) and 7(b) for NG and AD, respectively. The amount of the transferable hydrogen in NG would be sufficient to effectively quench the radicals formed and induce softening, whereas the AD exhibits no softening possibly due to the less amount of transferable hydrogen.
The authors proposed an original approach to quantify transferable hydrogen of coal upon heating. Coal pyrolysis involves condensation reactions of aromatic ring systems that induce hydrogen formation and aromatic cluster growth. The 13C- and 1H-NMR data for the coal and the heat treated char samples made the quantifications of the average size of aromatic clusters. The amount of hydrogen possibly generated by the aromatic growth was stoichiometrically estimated. The consideration on the fate of the hydrogen formed within the pyrolyzing coal suggested the possible conversion routes of hydrogen in solid/liquid phases, including the hydrogen used for hydro-deoxygenation and the hydrogen transferred for stabilizing radicals formed by thermal decomposition. The amount of hydrogen consumed for hydro-deoxygenation can be estimated from the difference between the amounts of pyrolytic water released into gas phase and water formed by hydroxyls condensation. With the balance, it was eventually possible to estimate the amount of the transferable hydrogen. According to the proposed approach, it can be revealed that the bituminous coal NG, showing plasticity, had 3.5 mol/kg-coal transferable hydrogen, whereas sub-bituminous coal AG, a non-caking coal, exhibited much less transferable hydrogen, 1.3 mol/kg-coal during pyrolysis up to 500°C within the limits of the present experimental conditions and analytical accuracies.
A part of this work was financially supported by The Iron and Steel Institute of Japan for 2015–2018.