2020 Volume 60 Issue 4 Pages 640-648
2020 Volume 60 Issue 4 Pages 640-648
Thermodynamic behavior of nitrogen in Fe–Cr–Ti–Al–Si–N alloy melts was investigated by measuring the nitrogen solubility and solubility product of TiN and AlN by the metal/gas and metal/nitride/gas equilibration techniques at 1823–1873 K, respectively. The nitrogen solubility data measured in Fe–Cr–Ti, Fe–Cr–Si, Fe–Ti–Al and Fe–Cr–Al alloy melts was thermodynamically analyzed to determine the second-order cross-product parameters of Cr–Ti, Cr–Si, Al–Ti and Cr–Al on nitrogen in liquid iron using Wagner’s formalism. By considering the cross-product effect on nitrogen determined in the present study, the effects of the alloying elements on the solubility product of TiN and AlN in the multicomponent Fe–Cr–Ti, Fe–Ti–Al and Fe–Cr–Al alloy melts were successfully reproduced over the wide temperature range.
Titanium in ferritic stainless steel is well known to play an important role in enhancing the mechanical properties by the grain refinement.1) The small size titanium nitride particles formed during solidification act as the heterogeneous nucleation sites of delta-ferrite.2) Therefore, in order to control the solidification structure of ferritic stainless steels, it is essential to have accurate thermodynamic information of the N solubility and nitride formation in multicomponent Fe–Cr–Ti–Al–Si–N alloy melt over the wide temperature range from steelmaking to the liquidus temperature.
In the past decade, the present research group has studied the thermodynamics of N solubility and nitride formation in all sub-systems3,4,5,6,7,8,9,10) of the Fe–Cr–Ti–Al–Si–N alloy melt which was aimed at developing a thermodynamic database for the refining of ferritic stainless steel. The present study can complete the thermodynamic study of N in the typical ferritic stainless steel by merging the authors’ recent studies on the Fe–Cr–N,3) Fe–Ti–N,4) Fe–Al–N,5) Fe–Si–N,6) Fe–Cr–Ti–N,7) Fe–Cr–Al–N,8) Fe–Ti–Al–N,9) Fe–Ti–Si–N10) and Fe–Al–Si–N6) systems with a modification for higher-order systems. According to our most recent study,6) it was also found that the simultaneous effect of alloying elements on N in liquid iron could exist in the multicomponent system at high alloy concentration region. In such a case, it can cause a poor prediction of both N solubility and nitride solubility product without considering the simultaneous effect.
Therefore, in the present study, the N solubility in Fe–Cr–Ti, Fe–Cr–Si, Fe–Ti–Al and Fe–Cr–Al alloy melts was measured under various nitrogen partial pressures at 1823–1873 K over the wide concentration range to determine the simultaneous effects of two different alloying elements on N in liquid iron. These interactions between N and alloying elements in liquid iron were expressed as the second-order cross-product parameters of N using Wagner’s formalism.11) The validity of the interaction parameters determined in the present study was checked by measuring the N solubility for a typical ferritic stainless steel melt of Fe-18 mass%Cr-0.4 mass%Ti-0.3 mass%Al-0.2 mass%Si–N alloy at 1823–1973 K. The solubility product of TiN and AlN in Fe–Cr–Ti, Fe–Ti–Al and Fe–Cr–Al alloy melts was also measured at 1823–1873 K. The solubility product data was thermodynamically analyzed by considering the second-order cross-product parameters of N to verify the accuracy of the first-order interaction parameters between alloying elements (Cr–Ti, Ti–Al and Cr–Al) in liquid iron determined in the authors’ recent studies.7,8,9)
The gas-liquid metal and the gas-liquid metal-nitride equilibration experiments were carried out to determine the N solubility and solubility product of TiN or AlN in liquid iron alloys containing Cr, Ti, Al and Si, respectively. Five hundred grams of high purity electrolytic iron was melted in an Al2O3 crucible (outer diameter (OD): 56 mm, inner diameter (ID): 50 mm, height (H): 96 mm) using a 15 kW/30 kHz high frequency induction furnace, and the melt temperature was directly measured by a Pt/Pt-13 mass%Rh thermocouple sheathed with an 6 mmOD alumina tube immersed in the melt. After the melt temperature was reached to a desired value, the Ar-10%H2 gas was blown onto the melt surface at a high flow rate of ~2 L/min to deoxidize the melt. After 2 hours of gas blowing, the oxygen content in the melt decreased to a value less than 20 mass ppm, and then the gas was switched to a mixture of N2 and Ar-10%H2 gases to keep the aimed nitrogen partial pressures. The flow rate of the gas mixture was 1 L/min. Detailed descriptions of the experimental apparatus and procedure are available in the authors’ recent study.7,9)
The simultaneous effects of two different alloying elements on N in liquid iron were determined by measuring the N solubility in Fe–Cr–Ti, Fe–Cr–Si, Fe–Ti–Al and Fe–Cr–Al melts at 1823–1873 K. After confirming the equilibrium N solubility in pure liquid iron under various nitrogen partial pressures from 0.03 to 0.1 atm, high purity Cr (99.95%purity), Ti (99.995%purity), Si (99.99%purity) or Al (99.99%purity) shots were added to liquid iron through an 18 mmOD quartz tube. After each alloy addition, a new equilibrium value of the N content was attained within 1 hour. This was confirmed by the sampling and in-situ analysis of N at 15 min intervals.
As a verification experiment, the N solubility in a multi-component Fe–Cr–Ti–Al–Si–N system of typical ferritic stainless steel were also measured as a function of melt temperature. After the equilibrium N solubility was attained for a Fe-18 mass%Cr-0.3 mass%Al-0.2 mass%Si alloy melt at 1823 K under a nitrogen partial pressure of 0.01 atm, Ti was added repeatedly up to 0.4 mass% while the melt temperature was increased to 1923 K.
The solubility product of TiN or AlN in Fe–Cr–Ti, Fe–Ti–Al and Fe–Cr–Al alloy melts was also measured by the addition of alloying elements after the formation of the nitride phases. For the Fe–Cr–Ti and Fe–Al–Ti systems, Ti shot was first added in pure liquid iron until a TiN layer was formed on the surface of the melts under a given nitrogen partial pressure. The formation of TiN in the melt could be confirmed by a sharp decrease in N content in the melt checked by the in-situ analysis of metal samples during the experiment. Then, Cr or Al shots were added repeatedly at 1823–1873 K. After each addition, a new TiN solubility equilibrium was attained within 1 h. For the Fe–Cr–Al and Fe–Ti–Al systems, Al shots were first added in pure liquid iron until a AlN layer was formed on the surface of the melt, and then Cr or Ti shots were added at 1823 K.
The metal sample of about 10 g was extracted by a 4 mmID quartz tube connected to a syringe (10 ml), and it was quenched rapidly in ice water. The metal samples were carefully cut for the chemical analysis. Cr, Ti, Al and Si in the metal sample were analyzed by the inductively coupled plasma atomic emission spectroscopy (ICP-AES, SPECTRO ARCOS) using appropriate standard solutions containing the same amount of Fe as the sample solutions. The analytical limit of the alloying elements in the metal sample was 5±1 mass ppm. The N and O contents in the metal sample were analyzed by the nitrogen/oxygen analyzer (LECO TC-600) with an accuracy of ±2 mass ppm.
The dissolution of N in liquid iron alloys can be written as
| (1) 12) |
| (2) |
The activity coefficient of N, fN in Fe–Cr–Ti–Al–Si–N melt can be expressed by Wagner’s formalism11) as the following relation using the interaction parameters:
| (3) |
In the authors’ recent studies, the
| System | Interaction parameter | Value (1873 K) | Temp.(K)/[%i] range | pN2 (atm) | Ref. |
|---|---|---|---|---|---|
| Fe–Cr–N | −147.8/T + 0.019 (−0.06) | 1873–1973 [%Cr] < 30 | 0.015–0.97 | 3 | |
| −2.58/T + 0.0021 (0.0007) | |||||
| Fe–Ti–N | −0.21 | 1823–1973 [%Ti] < 0.52 | 0.05–0.3 | 4 | |
| 0 | |||||
| 0.048 | |||||
| 0 | |||||
| logKTiN | −12740/T + 4.06 (−2.742) | ||||
| Fe–Al–N | 0.017 | 1823–1973 [%Al] < 4.5 | 0.2–1 | 5 | |
| 0 | |||||
| 0.043 | |||||
| 0 | |||||
| logKAlN | −15850/T + 7.03 (−1.434) | ||||
| Fe–Si–N | 0.047 | 1823–1873 [%Si] < 13 | 0.4–0.8 | 6 | |
| 0.0013 | |||||
| Fe–Cr–Ti–N | 0 | 1823 [%Cr] < 14, [%Ti] < 0.083 | 0.03–0.08 | Present study | |
| 406.7/T − 0.1933 (0.024) | 1823–1923 [%Cr] < 30, [%Ti] < 1.5 | 0.01–0.05 | 7 | ||
| −20.6/T + 0.011 (0) | |||||
| Fe–Cr–Al–N | −0.003 | 1823–1873 [%Cr] < 30, [%Al] < 1.0 | 0.04 | Present study | |
| 0.017 | 1823–1973 [%Cr] < 26, [% Al] < 3.2 | 0.1–0.5 | |||
| 0 | |||||
| Fe–Cr–Si–N | 0 | 1823 [%Cr] < 20, [%Si] < 1.4 | 0.09 | Present study | |
| Fe–Ti–Al–N | 0 | 1823 [%Ti] < 0.066, [%Al]< 0.28 | 0.1 | Present study | |
| −0.024 | 1823–1973 [%Ti] < 0.55, [%Al] < 1.6 | 0.1–0.2 | |||
| 0 | |||||
| −0.011 | 1823 [%Ti] < 0.14, [%Al] < 2.0 | 0.1 | |||
| 0 | |||||
| Fe–Ti–Si–N | 0 | 1823–1873 [%Ti] < 0.083, [%Si] < 2.3 | 0.05–0.1 | 10 | |
| −0.038 | 1823–1923 [%Ti] < 0.36, [%Si] < 2.2 | 0.1–0.7 | |||
| 0 | |||||
| Fe–Al–Si–N | 0 | 1823–1873 [%Al] < 1.1, [%Si] < 1.8 | 0.1–0.3 | 6 | |
| 0.037 | 1823–1923 [%Al] < 1.7, [%Si] < 1.5 | 0.3–1 | |||
| 0 |
| System | Temp. (K) | pN2 (atm) | [%Cr] | [%Ti] | [%Al] | [%Si] | [%N] | [%O] |
|---|---|---|---|---|---|---|---|---|
| Fe–Cr–Ti–N | 1823 | 0.03 | 2.65 | 0 | 0 | 0 | 0.0109 | 0.0008 |
| 2.78 | 0.02 | 0 | 0 | 0.0110 | 0.0012 | |||
| 2.71 | 0.03 | 0 | 0 | 0.0109 | 0.0015 | |||
| 2.74 | 0.05 | 0 | 0 | 0.0111 | 0.0015 | |||
| 2.65 | 0.07 | 0 | 0 | 0.0115 | 0.0014 | |||
| 2.82 | 0.08 | 0 | 0 | 0.0114 | 0.0008 | |||
| 0.08 | 14.51 | 0 | 0 | 0 | 0.0699 | 0.0026 | ||
| 14.57 | 0.02 | 0 | 0 | 0.0701 | 0.0031 | |||
| 14.25 | 0.04 | 0 | 0 | 0.0712 | 0.0026 | |||
| 14.21 | 0.05 | 0 | 0 | 0.0724 | 0.0027 | |||
| Fe–Cr–Si–N | 1823 | 0.09 | 4.70 | 0 | 0 | 0.03 | 0.0257 | 0.0021 |
| 4.79 | 0 | 0 | 0.15 | 0.0257 | 0.0018 | |||
| 4.71 | 0 | 0 | 0.25 | 0.0258 | 0.0012 | |||
| 4.72 | 0 | 0 | 0.35 | 0.0255 | 0.0014 | |||
| 9.57 | 0 | 0 | 0.39 | 0.0442 | 0.0020 | |||
| 9.63 | 0 | 0 | 0.50 | 0.0430 | 0.0018 | |||
| 9.50 | 0 | 0 | 0.60 | 0.0417 | 0.0014 | |||
| 9.52 | 0 | 0 | 0.69 | 0.0416 | 0.0015 | |||
| 14.77 | 0 | 0 | 0.66 | 0.0695 | 0.0024 | |||
| 14.62 | 0 | 0 | 0.84 | 0.0682 | 0.0025 | |||
| 14.71 | 0 | 0 | 0.94 | 0.0676 | 0.0018 | |||
| 14.61 | 0 | 0 | 0.90 | 0.0672 | 0.0021 | |||
| 19.69 | 0 | 0 | 1.04 | 0.107 | 0.0028 | |||
| 19.60 | 0 | 0 | 1.15 | 0.105 | 0.0025 | |||
| 19.56 | 0 | 0 | 1.34 | 0.103 | 0.0026 | |||
| 19.61 | 0 | 0 | 1.43 | 0.104 | 0.0026 | |||
| Fe–Al–Ti–N | 1823 | 0.1 | 0 | 0.02 | 0.28 | 0 | 0.0136 | 0.0009 |
| 0 | 0.03 | 0.28 | 0 | 0.0134 | 0.0014 | |||
| 0 | 0.04 | 0.28 | 0 | 0.0137 | 0.0013 | |||
| 0 | 0.05 | 0.28 | 0 | 0.0137 | 0.0011 | |||
| 0 | 0.07 | 0.27 | 0 | 0.0138 | 0.0008 | |||
| Fe–Cr–Al–N | 1823 | 0.1 | 9.38 | 0 | 0 | 0 | 0.0446 | 0.0018 |
| 14.00 | 0 | 0 | 0 | 0.0726 | 0.0014 | |||
| 14.09 | 0 | 0.13 | 0 | 0.0725 | 0.0013 | |||
| 13.66 | 0 | 0.20 | 0 | 0.0724 | 0.0011 | |||
| 13.50 | 0 | 0.26 | 0 | 0.0710 | 0.0009 | |||
| 18.52 | 0 | 0.33 | 0 | 0.1080 | 0.0009 | |||
| 18.01 | 0 | 0.33 | 0 | 0.1070 | 0.0011 | |||
| 17.55 | 0 | 0.70 | 0 | 0.1050 | 0.0008 | |||
| 1873 | 0.04 | 8.92 | 0 | 0 | 0 | 0.0279 | 0.0008 | |
| 8.80 | 0 | 0.19 | 0 | 0.0278 | 0.0016 | |||
| 8.88 | 0 | 0.41 | 0 | 0.0272 | 0.0016 | |||
| 16.38 | 0 | 0.34 | 0 | 0.0575 | 0.0015 | |||
| 16.25 | 0 | 0.55 | 0 | 0.0563 | 0.0011 | |||
| 23.09 | 0 | 0.49 | 0 | 0.0862 | 0.0017 | |||
| 22.61 | 0 | 0.66 | 0 | 0.0908 | 0.0017 | |||
| 29.55 | 0 | 0.55 | 0 | 0.1337 | 0.0013 | |||
| 28.70 | 0 | 0.87 | 0 | 0.1497 | 0.0022 | |||
| 28.50 | 0 | 1.00 | 0 | 0.1490 | 0.0016 | |||
| Fe–Cr–Ti–Al–Si–N | 1823 | 0.01 | 18.39 | 0 | 0.29 | 0.37 | 0.0365 | 0.0020 |
| 18.27 | 0.04 | 0.29 | 0.37 | 0.0373 | 0.0011 | |||
| 1873 | 18.45 | 0.07 | 0.29 | 0.41 | 0.0350 | 0.0016 | ||
| 18.43 | 0.10 | 0.29 | 0.38 | 0.0346 | 0.0005 | |||
| 18.46 | 0.14 | 0.28 | 0.38 | 0.0357 | 0.0019 | |||
| 1923 | 18.89 | 0.14 | 0.29 | 0.37 | 0.0330 | 0.0019 | ||
| 18.50 | 0.17 | 0.28 | 0.36 | 0.0333 | 0.0010 | |||
| 18.31 | 0.21 | 0.28 | 0.38 | 0.0337 | 0.0014 |
Using the relation shown in Eq. (3), the second-order cross-product parameters on N in liquid iron,
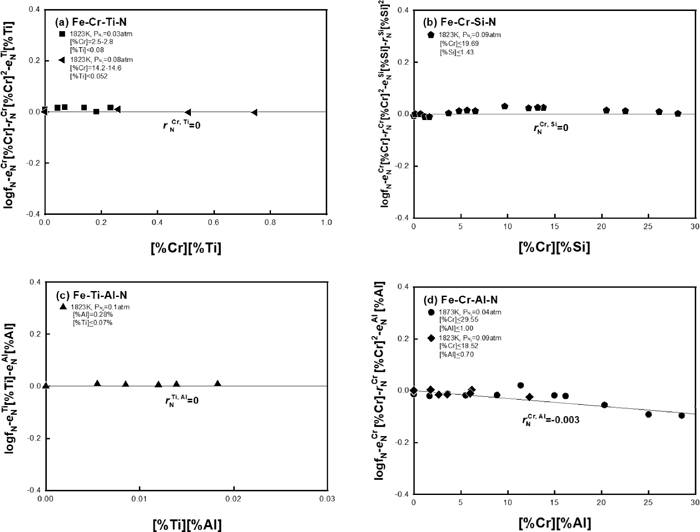
Relation of Eq. (3) to determine the value of (a)
Using all parameters used and determined in the present study for the Fe–Cr–Ti–Al–Si–N system, the N solubility in the multicomponent alloy melts can be calculated at various compositions and temperatures by the following relation obtained from Eq. (3):
| (4) |
In order to check the validity of interaction parameters determined in the present study, a verification experiment was carried out by measuring the N solubility in Fe-18 mass%Cr-0.3 mass%Al-0.4 mass%Si–Ti melt under a nitrogen partial pressure of 0.01 atm. The N solubility was measured by adding Ti in the melt with increasing temperature from 1823 to 1923 K at every 50 K. The experimental results are also summarized in Table 2. Figure 2 compares the experimental results of N solubility with the predicted values in the melt as a function of Ti content at different temperatures. The present result reproduces the N solubility successfully over the wide temperature range from 1823 to 1923 K.
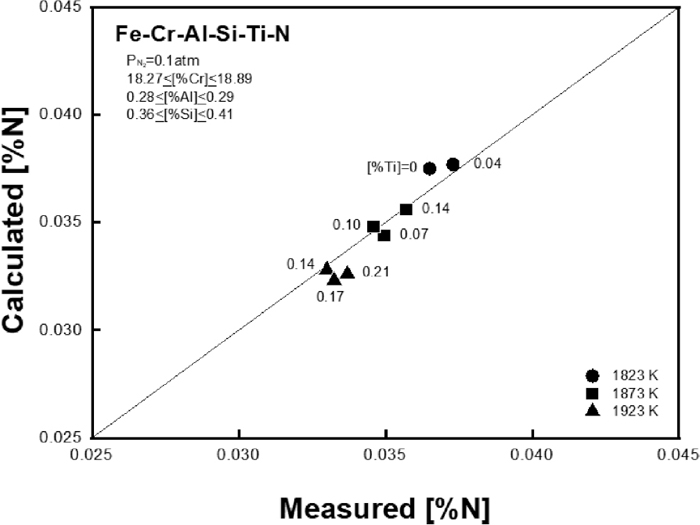
Correlation between calculated and measured N solubility in Fe–Cr–Al–Si–Ti–N melt.
Ti and Al can form their nitride phases such as TiN and AlN, respectively, at critical concentrations in Fe–Cr alloy melt at a given nitrogen content. The effect of Cr on the solubility product of those nitrides in liquid iron has been measured in the temperature range from 1873 to 1973 K by the authors’ previous studies.7,8) However, a description of the solubility product of nitrides at lower temperature range near the melting point of Fe–Cr alloys is needed for more accurate prediction of the nitride formation during cooling and solidification. For this purpose, the solubility product of nitride phases in Fe–Cr–Ti, Fe–Al–Ti and Fe–Cr–Al systems were measured at 1823–1873 K to clarify the temperature dependence of the nitride formation reactions in Fe–Cr–Ti–Al alloy melt. The experimental results of TiN and AlN solubility product in the melts were summarized in Table 3, and they are plotted in Figs. 3 and 4 together with the authors’ previous results at higher temperatures.
| System | Temp.(K) | pN2 (atm) | [%Cr] | [%Ti] | [%Al] | [%Si] | [%N] | [%O] | Nitride saturation |
|---|---|---|---|---|---|---|---|---|---|
| Fe–Cr–Ti | 1823 | 0.03 | 2.80 | 0.25 | 0 | 0 | 0.0062 | 0.0012 | TiN |
| 2.72 | 0.40 | 0 | 0 | 0.0040 | 0.0016 | TiN | |||
| 3.65 | 0.39 | 0 | 0 | 0.0044 | 0.0008 | TiN | |||
| 10.93 | 0.30 | 0 | 0 | 0.0085 | 0.0007 | TiN | |||
| 16.02 | 0.26 | 0 | 0 | 0.0127 | 0.0016 | TiN | |||
| 20.75 | 0.22 | 0 | 0 | 0.0181 | 0.0011 | TiN | |||
| 23.39 | 0.16 | 0 | 0 | 0.0256 | 0.0019 | TiN | |||
| Fe–Ti–Al | 1823 | 0.1 | 0 | 0.09 | 0.28 | 0 | 0.0128 | 0.0008 | TiN |
| 0 | 0.09 | 0.65 | 0 | 0.0135 | 0.0008 | TiN | |||
| 0 | 0.10 | 1.10 | 0 | 0.0120 | 0.0012 | TiN | |||
| 0 | 0.11 | 1.57 | 0 | 0.0120 | 0.0016 | TiN | |||
| 0.1 | 0 | 0 | 1.68 | 0 | 0.0097 | 0.0006 | AlN | ||
| 0 | 0.05 | 1.92 | 0 | 0.0088 | 0.0008 | AlN | |||
| 0 | 0.06 | 1.94 | 0 | 0.0089 | 0.0010 | AlN | |||
| 0 | 0.10 | 1.87 | 0 | 0.0095 | 0.0029 | AlN | |||
| 0 | 0.12 | 1.91 | 0 | 0.0098 | 0.0011 | AlN | |||
| 0 | 0.14 | 1.96 | 0 | 0.0091 | 0.0021 | AlN | |||
| 1873 | 0.1 | 0 | 0.21 | 0 | 0 | 0.0085 | 0.0011 | TiN | |
| 0 | 0.26 | 0.29 | 0 | 0.0072 | 0.0007 | TiN | |||
| 0 | 0.28 | 0.58 | 0 | 0.0067 | 0.0008 | TiN | |||
| 0 | 0.34 | 0.86 | 0 | 0.0060 | 0.0002 | TiN | |||
| 0 | 0.41 | 1.16 | 0 | 0.0054 | 0.0013 | TiN | |||
| 0 | 0.48 | 1.48 | 0 | 0.0048 | 0.0016 | TiN | |||
| Fe–Cr–Al | 1823 | 0.09 | 16.91 | 0 | 0.99 | 0 | 0.0906 | 0.0015 | AlN |
| 17.72 | 0 | 1.24 | 0 | 0.0746 | 0.0017 | AlN | |||
| 0.48 | 0 | 0 | 1.34 | 0 | 0.0141 | 0.0009 | AlN | ||
| 4.83 | 0 | 0.98 | 0 | 0.0340 | 0.0006 | AlN | |||
| 9.56 | 0 | 0.45 | 0 | 0.1110 | 0.0010 | AlN | |||
| 14.36 | 0 | 0.44 | 0 | 0.1653 | 0.0013 | AlN |
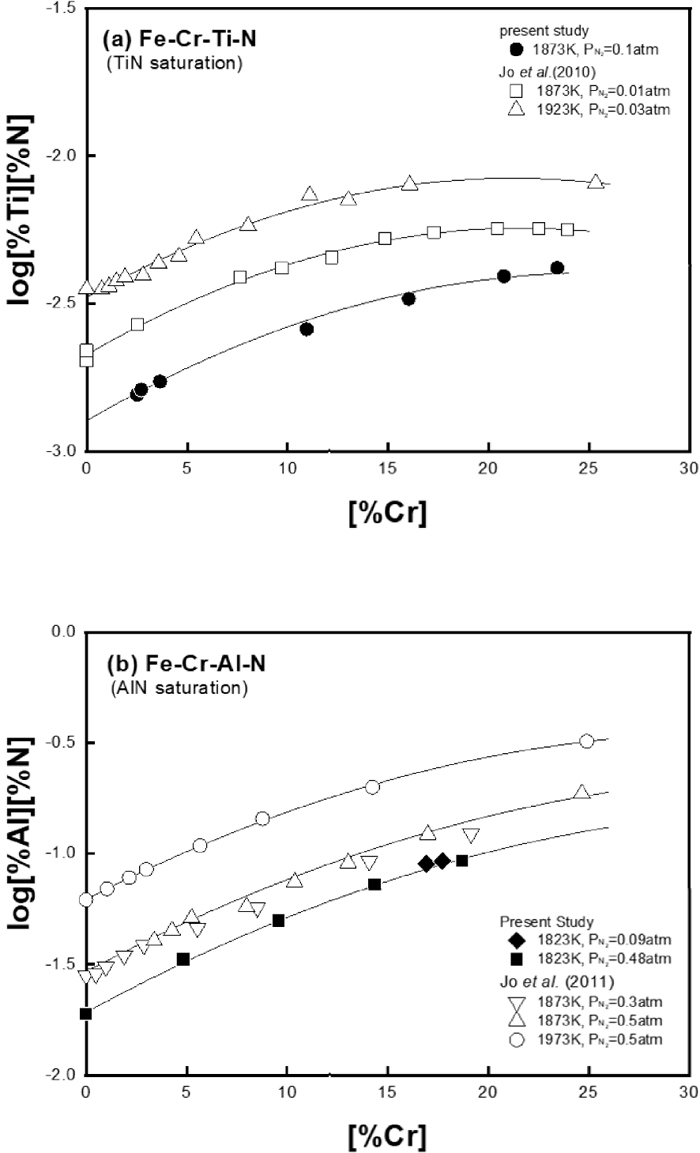
Effect of Cr on (a) TiN solubility product and (b) AlN solubility product in liquid iron.
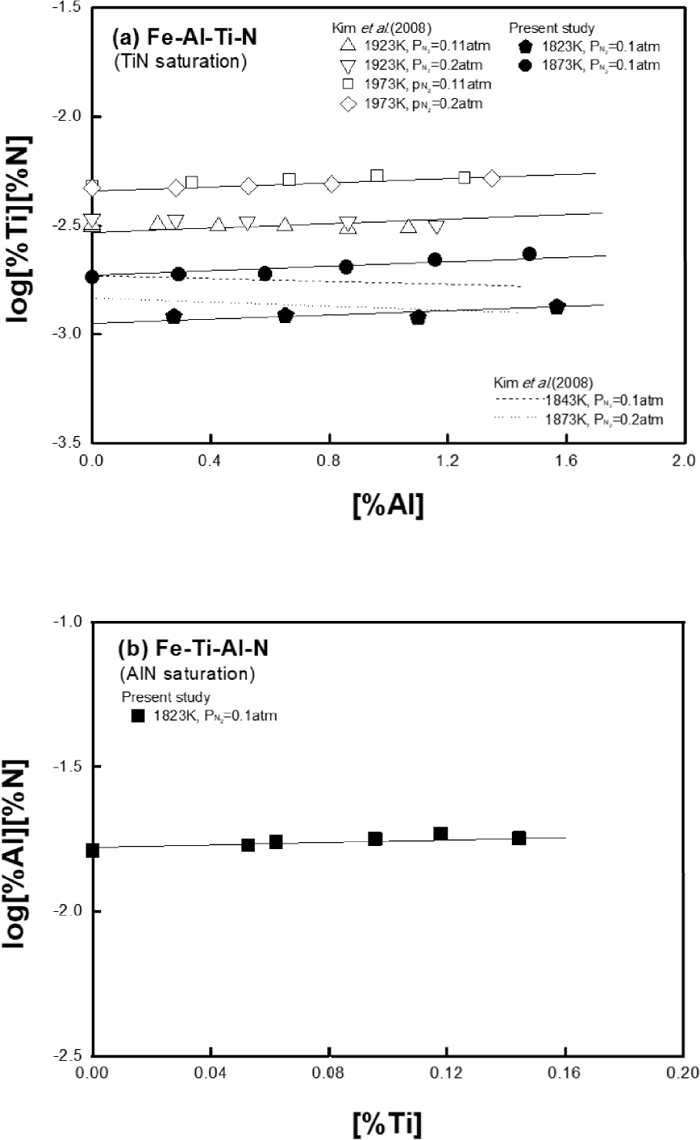
Effect of (a) Al on TiN solubility product and (b) Ti on AlN solubility product in liquid iron.
Figure 3 shows the effects of Cr on TiN and AlN solubility product in liquid iron, respectively, at different temperatures. Both solubility products of TiN and AlN increased with the addition of Cr. As shown Figs. 3(a) and 3(b), the present results of TiN and AlN solubility product measured at 1823 K have good correlation with data measured by the authors’ recent studies7,8) at higher temperatures.
Figure 4(a) shows the effect of Al addition on TiN solubility product in liquid iron at different temperatures. In authors’ previous study,9) the effect of Al on TiN solubility product was different at high and low temperatures as shown in the figure. The discrepancy at low temperatures was already discussed and revised elsewhere for the Fe–Ti–N ternary system.4) Therefore, in the present study, the effect of Al on TiN solubility product in liquid iron was restudied at 1823 and 1873 K and compared with the authors’ previous data in Fig. 4(a). Also, the effect of Ti on AlN solubility product was measured at 1823 K as shown in Fig. 4(b) to check if the interaction between Ti and Al in liquid iron is consistent regardless of type of nitride phase formed in the same alloy system.
In order to thermodynamically analyze the formation of TiN and AlN in Fe–Cr–Ti–Al–Si–N alloy melts, the following equilibrium reactions for the dissolution of pure solid TiN and AlN in liquid iron were considered with the Gibbs energy of each reaction determined in the authors’ recent studies.4,5)
| (5) 4) |
| (6) |
| (7) 5) |
| (8) |
At a given temperature, the solubility product of TiN and AlN, log[%Ti][%N] and log[%Al][%N] in Eqs. (6) and (8) depends on fTi, fAl and fN values in liquid steel. For an Fe–Cr–Ti–Al–Si–N melts, those activity coefficients can be expressed as the following relations using the Wagner’s formalism.11)
| (9) |
| (10) |
| (11) |
Figure 5 shows the value of
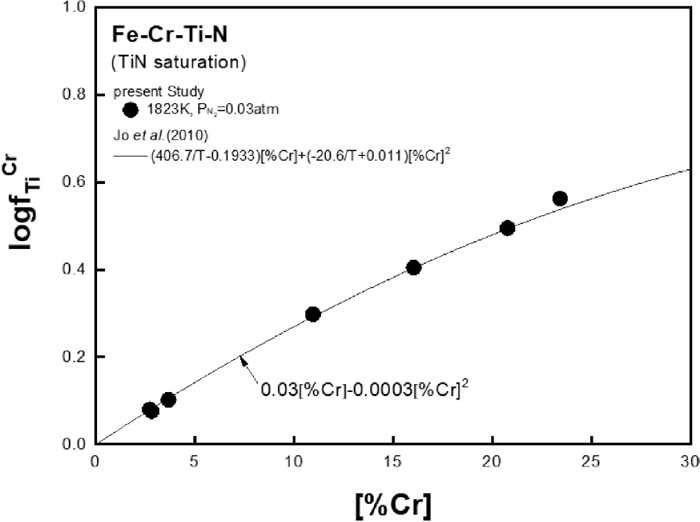
Relation of
Figure 6 shows the value of
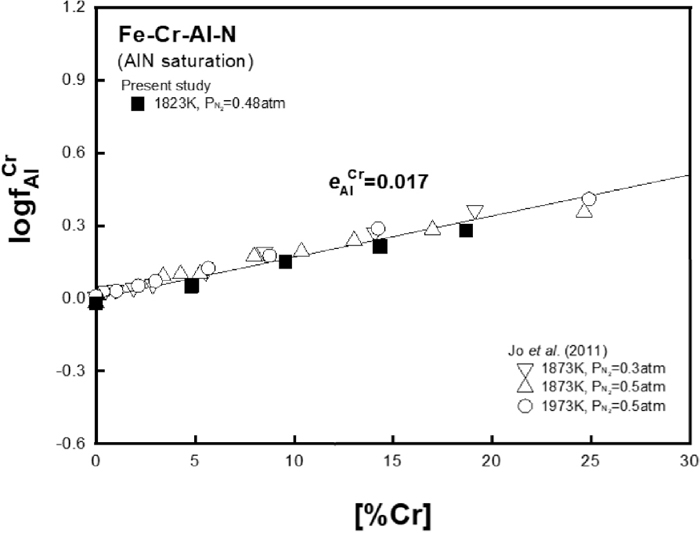
Relation of
The
Figure 7 shows the value of
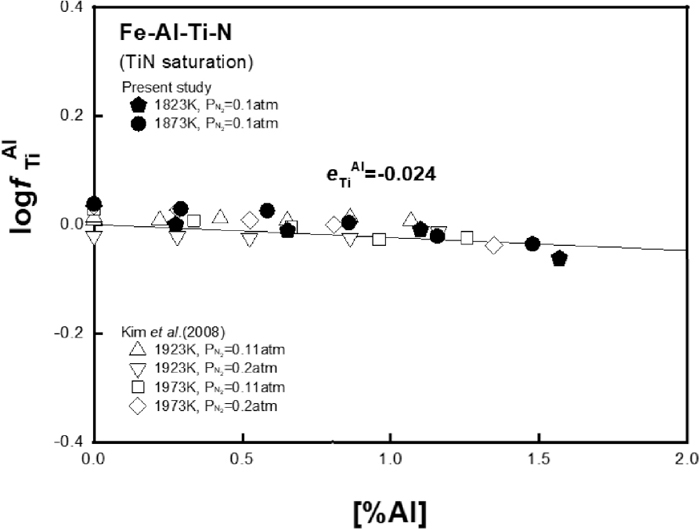
Relation of
Figure 8 shows the
| (12) |
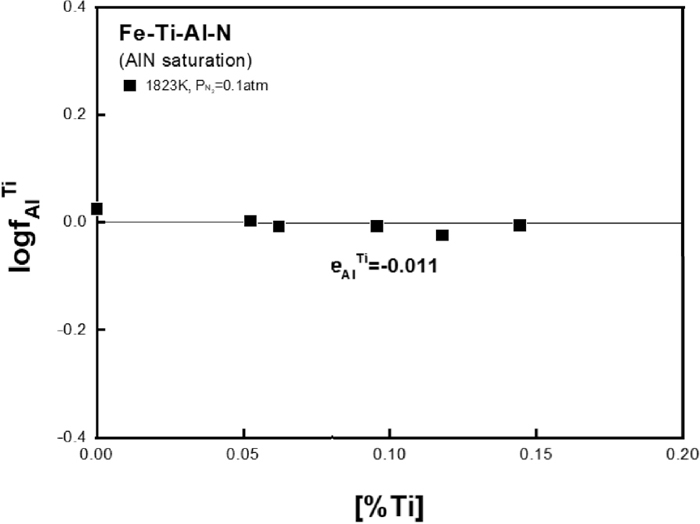
Relation of
The value of
The interaction parameters determined in the present study were summarized in Table 1 along with all parameters used in this study. Using the parameters, one can calculate the contour lines of critical Ti and N contents for the onset of TiN inclusion formation in Fe–Cr–Ti–Al–Si–N alloy melt as functions of melt composition and temperature. As shown in Fig. 9, the TiN solubility diagram for a commercial ferritic stainless steel composition of Fe-18 mass%Cr-0.3 mass%Al-0.3 mass%Si–Ti alloy was constructed at the different temperatures from 1823 to 1923 K. Figure 9 can be used for quick check of critical N and Ti content to avoid the formation of TiN in the ferritic stainless steels. This type of diagram can be also predicted for different temperatures, nitrogen partial pressures, and composition to track the nitride formation during the steelmaking process of ferritic stainless steels.

TiN solubility diagram for a Fe-18 mass%Cr-0.3 mass%Al-0.3 mass%Si–Ti–N melt.
In the present study, the effect of cross-products of alloying elements on nitrogen in liquid Fe–Cr–Ti–N, Fe–Cr–Si–N, Fe–Ti–Al–N and Fe–Cr–Al–N melts were determined at 1823–1873 K. By taking into account the simultaneous effects determined in the present study, the interaction parameters among Cr, Al and Ti were newly determined. These parameters could be used to accurately predict the N solubility and solubility product of TiN and AlN in multicomponent Fe–Cr–Ti–Al–Si system. The main findings of this study can be summarized as follows:
(1) The second-order cross-product effects of alloying elements on nitrogen in Fe–Cr–Ti, Fe–Cr–Si, Fe–Ti–Al and Fe–Cr–Al alloy melts were determined as:
(2) The first- and second-order interaction parameters of alloying elements on Ti and Al in Fe–Cr–Ti, Fe–Ti–Al and Fe–Cr–Al alloy melts were determined as:
This paper was supported by Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (MOTIE) (P0002019, The Competency Development Program for Industry Specialist).