2021 Volume 61 Issue 1 Pages 158-166
2021 Volume 61 Issue 1 Pages 158-166
The gasification behaviors of coke in a blast furnace with and without H2 were studied by thermodynamic calculations and high-temperature simulation experiments, and the change in the coke porosity was also studied. The results show that with the decrease in φ(CO)/φ(H2), the temperature range of C gasification decreases and moves to the low-temperature zone. In the absence of H2, the increase in φ(CO) increases the Ri, RC. In the presence of H2, φ(CO) and Ri increase, whereas the RC, decreases. With the increase in φ(CO) and φ(H2), the reduction of iron oxide tends to be carried out in the low-temperature zone, and the φ(CO2) and φ(H2O) produced in the high-temperature zone decrease, which is conducive to reducing the consumption of coke. The presence of H2 intensifies the gasification of coke. The presence of H2 aggravates the increase of coke porosity in the low temperature region, but it reduces the internal porosity in the high temperature region.
The distribution of high-quality coking coal in China is not uniform, and the reserves are small.1,2) The shortage of coking resources directly affects the sustainable development of blast furnace ironmaking, and the impact of the shortage on blast furnace operations is becoming increasingly obvious. Moreover, with the development of large-scale blast furnaces and smelting technology, the coke ratio in blast furnaces continues to decrease, and the time the coke resides in blast furnaces is being prolonged. The quality requirement of coke is increasing.3,4) Therefore, rationally using coke resources has become a key issue that is restricting the development of blast furnace ironmaking. Researchers are paying increasing attention to the study of multiphase reaction mechanisms and the property evolution of coke in blast furnaces.
When coke enters a blast furnace, it generates gasification, smelting reduction, carburization and combustion reactions in the tuyere area as the temperature increases. The results have shown5,6) that the degree of coke gasification in a blast furnace has an important influence on the deterioration of coke properties. The micropore structure of coke after a reaction directly affects the skeleton function of the coke in the whole blast furnace, the coke strength and the blast furnace permeability. A substantial amount of research has been performed on the kinetics of the coke gasification reaction and changes in the microstructure after coke reactions. It was noted that7,8,9) the gasification reaction of coke conforms to the model of unreacted nuclear shrinkage and that the effective diffusion activation energy of coke gasification is larger than the apparent activation energy of coke gasification; as a result, the relative resistance of interfacial chemical reactions increases with an increase in the reaction temperature, and with an increase in the reaction temperature, the rate-controlled region of the interfacial chemical reactions increases during coke gasification. Yang et al.10) noted that the specific surface area of coke increased when the volume fraction of CO2 was low, and it increased first and then decreased when the volume fraction of CO2 was high. Cui et al.11) and Fang et al.12) studied the effect of CO2 on the high-temperature compressive strength of coke, which decreased with increasing temperature, CO2 volume fraction and dissolution rate; during the low-temperature stage, the dissolution reaction of the coke was mainly on the surface, and the compressive strength was relatively high, whereas during the high-temperature stage, the dissolution reaction of the coke gradually diffused into the interior so that the pore wall of the coke was fractured, perforated and denuded, and the compressive strength of the coke was relatively low. The influence of H2O and CO2 on the microstructure of the coke was also compared and analyzed. Compared with CO2, H2O better promoted the gasification reaction of coke.13,14,15,16,17) Xu et al.18) indicated that the coke gasification can increase the ordered degree due to the consumption of active carbon atoms, the ordered degree of carbon crystal in the reacted coke with H2O is higher than that in the reacted coke with CO2, and H2O can consume more isotropy than CO2 due to its small molecule size and fast reaction rate. Under high temperature conditions, the reaction of coke and H2O occurred mostly on the surface of the coke, had less erosion on the central structure and had a certain protective effect on the strength of the coke.19,20)
The above studies mostly used pure CO2 and pure H2O to study the gasification reaction of coke at a constant temperature. During actual production processes that occur in blast furnaces, the CO2 and H2O involved in the coke gasification reaction are provided by the reduction of iron ore.21,22) The reduction rate of iron oxide varies with the gas composition and temperature, and the volume fractions of CO2 and H2O produced at different temperatures and gas compositions are also different. Furthermore, the changes in the coke gasification reaction rate and pore structure are affected; that is, synergistic reaction processes of ore reduction and coke gasification exist in the blast furnace. At present, research on this aspect is relatively scarce. As a result of the development of hydrogen-rich blast furnace technology, it was found that the appropriate hydrogen-rich content of blast furnace is 10%–15%.23,24) When H2 is added to the blast furnace gas, competition exists between H2 and CO for ore reduction. The volume fraction of CO2 and H2O that is produced also changes, and the effect of this change on the gasification process of coke has not been studied. Based on this, the change in the gasification behaviors of coke in the blast furnace and the porosity of the coke under the conditions with and without H2 are studied by thermodynamic calculation and high-temperature simulation experiments. The results reflect the deterioration process of coke in a blast furnace and provide a reliable theoretical reference for blast furnace production.
The reduction reaction of iron oxide and the gasification reaction of coke in the blast furnace are shown in Table 1, wherein the reaction thermodynamic data are from reference.25)
| No. | Chemical reaction equations | |
|---|---|---|
| Eq. (1) | −42639−47.39T | |
| Eq. (2) | 26044−30.44T | |
| Eq. (3) | −18628+22.17T | |
| Eq. (4) | −8287+9.993T | |
| Eq. (5) | −11917−75.52T | |
| Eq. (6) | 61473−62.88T | |
| Eq. (7) | 16826−10.30T | |
| Eq. (8) | 29369−25.07T | |
| Eq. (9) | 166500−171T | |
| Eq. (10) | 133100−141.63T |
Figure 1 shows the fork-shaped curves of iron oxide reduction under different φ(CO)/φ(H2). The φ(CO)/φ(H2) is the volume fraction ratio of CO and H2 in the initial atmosphere of the reaction, where the sum of φ(CO) and φ(H2) is 100%. The ordinate in Fig. 1 is the percentage of the volume fraction of (CO+H2) in the total gas (H2+CO+H2O+CO2) when the reaction reaches equilibrium. As shown in Fig. 1, when the temperature is lower than 1092 K, with the increase of φ(H2) in the gas, the concentration of the remaining reduction gas increases when the reaction reaches equilibrium, which is not conducive to the reduction of iron oxides. When the temperature is higher than 1092 K, with the increase of φ(H2) in the gas, the concentration of the remaining reduction gas decreases when the reaction reaches equilibrium, which is conducive to the reduction of iron oxides. However, in the actual process, because H2 molecule is far smaller than CO molecule, the kinetic conditions of H2 reduction of iron oxide process are better than CO, so the reduction capacity of H2 is still strong when it is lower than 1092 K.26,27) That is, the increase in φ(H2) in gas can effectively promote the reduction of iron oxide.

Fork-shaped curves of iron oxide reduction under different φ(CO)/φ(H2). (Online version in color.)
CO2 and H2O will be produced in the reduction process of iron oxide. CO2 and H2O will react with coke for gasification. With the decrease in the ratio of φ(CO)/φ(H2) in the gas, the φ(H2O) produced will increase gradually. The carbon consumption of the coke gasification process under different φ(CO2)/φ(H2O) conditions is shown in Fig. 2. As shown in Fig. 2, with increasing temperature, the gasification rate of C increases when the reaction reaches equilibrium. With increasing φ(H2O), the gasification rate of C increases, and the initial gasification temperature of C decreases. This shows that the increase in φ(H2O) will promote the consumption of coke. There is water gas shift reaction in the reaction system. The reaction equations are shown in Eqs. (11) and (12), respectively. According to the Gibbs free energy expressions of Eqs. (11) and (12), Eq. (11) occurs when the temperature is lower than 1091 K, and the reaction is gradually inhibited with increasing temperature. The occurrence of Eq. (11) promotes the conversion of H2O in the system, increases the amount of φ(CO2)/φ(H2O), and inhibits the gasification of coke. Equation (12) occurs when the temperature is higher than 1091 K, and the reaction is promoted gradually with increasing temperature. The occurrence of Eq. (12) promotes the increase of φ(H2O) in the system, which makes the decrease of φ(CO2)/φ(H2O), which promotes the gasification of coke.
| (11) |
| (12) |

Relationships between gasification rate and temperature under different φ(CO2)/φ(H2O). (Online version in color.)
Figure 3 is the thermodynamic equilibrium diagram of the synergistic reaction of iron oxide reduction and C gasification under different φ(CO)/φ(H2). From Fig. 3, the temperature of the start and end reaction of C gasification can be obtained, as shown in Fig. 4. It can be seen from Fig. 4 that with the decrease of φ(CO)/φ(H2), the start and end temperatures of C gasification in the system decrease gradually. When φ(CO)/φ(H2) changes from 4:1 to 1:4, the start temperature of C gasification in the system decreases from 952 K to 940 K, which is 12 K lower; the end temperature decreases from 1014 K to 992 K, which is 22 K lower; and the temperature range increases from 62 K to 52 K. The results show that the decrease in φ(CO)/φ(H2) can make the temperature range of C gasification move to the low-temperature zone.

Synergistic effect of iron oxide reduction and C gasification. (Online version in color.)

Temperature range of C gasification.
It can be seen from the above calculation results that the change in H2 content in the blast furnace will directly affect the synergistic effect of iron oxide reduction and coke gasification. Thermodynamic calculation only gives the equilibrium state of the chemical reaction and obtains the law of the chemical reaction. However, the reaction of iron oxide reduction and coke gasification in a blast furnace is affected by the actual heating process and reaction dynamics conditions. Therefore, it is necessary to further study coke gasification under the actual conditions of a simulated blast furnace.
The sinter, pellets and coke used in the experiment are all from a blast furnace production site. The sizes of the sinter and pellets are 10–12.5 mm, and the size of the coke is 12–14 mm. The chemical composition of the sinter and pellets is shown in Table 2. The industrial analysis, coke reactivity and postreaction strength are shown in Table 3.
| Chemical compositions | TFe | FeO | SiO2 | CaO | MgO | Al2O3 | TiO2 |
|---|---|---|---|---|---|---|---|
| sinter | 56.56 | 9.33 | 5.13 | 10.54 | 2.33 | 2.32 | 0.18 |
| pellet | 61.91 | 0.7 | 6.58 | 3.0 | 1.53 | 0.84 | 0.087 |
| Name | Industrial analysis/% | Reactivity/% | Post-reaction strength/% | ||
|---|---|---|---|---|---|
| Volatile | Ash | C | |||
| Coke | 1.40 | 13.30 | 85.31 | 22.88 | 67.16 |
Figure 5 is a schematic diagram of the equipment used in the experiment. The equipment simulates the burden structure and reaction state of the blast furnace. The maximum temperature in the equipment can reach 1873 K, and the equipment is heated by six silicon-molybdenum bars. Temperature control is managed by the DK-1 resistance furnace control cabinet. The furnace tube is a Φ80×5×800 mm fused corundum tube. The crucible is a Φ58×5×110 mm graphite crucible. The reaction gas is introduced from the lower part of the furnace body, and the purity of the reaction gas is 99.99%.

Schematic diagram of the equipment. (Online version in color.)
Coke and iron ore are separately packed into crucibles with a composition of 22 g coke, 180 g sinter and pellets, and 22 g coke. The ratio of the sinter to pellets is 6:4. The heating process of the blast furnace is simulated according to the Chinese national standard method (GB/T 34211-2017: Iron ores-Method for determination of iron reduction softening drippinger performance under load).28) Below 1173 K, the heating rate is 10 K/min, and the heating rate is 2 K/min in the range of 1173–1373 K. Above 1373 K, the heating rate changes to 5 K/min until the sample reaches the predetermined temperature. The whole heating process is completed in N2–CO–H2 (5 L/min). The experiments were carried out in four different atmospheres: atmosphere a-φ(CO)=30%, φ(H2)=0%; atmosphere b-φ(CO)=40%, φ(H2)=0%; atmosphere c-φ(CO)=30%, φ(H2)=10%; and atmosphere d-φ(CO)=40%, φ(H2)=10%. When the temperature reaches the predetermined temperature (1173 K, 1273 K, 1313 K, 1473 K), the experiment is finished, and cooling occurs under N2 (5 L/min) protection. The cooled burden in the crucible is sorted, and the ore and coke are completely separated and weighed separately. The formulas for calculating the reduction degree of iron ore (Ri) and weight loss rate of coke (RC) are shown in Eqs. (13) and (14), respectively.29,30)
| (13) |
| (14) |
After the reaction, the coke was solidified in the solidifying agent solution. The coke was cut along the central plane by cutting equipment, and the cutting surface was polished. The coke pore structure automatic analysis system was used to identify the single point of the polished plane. The software in the system was used to analyze the parameters of the coke porosities at all the test points. The porosity of coke in different zones was obtained.
Figure 6 shows the effect of CO on iron ore reduction and coke gasification. As shown in Fig. 6(a), in the absence of H2, Ri and RC increase gradually with increasing φ(CO). The main reason for this result is that an increase in φ(CO) promotes the reduction of iron ore and an increase in φ(CO2). Especially in the temperature range of 1373 K to 1473 K, Ri increases substantially with an increase in φ(CO); the increase in φ(CO2) is large, and the thermodynamic and kinetic conditions of coke gasification in this temperature range are improved. Therefore, the RC value in this temperature range increases considerably with increasing φ(CO). Figure 6(b) shows that when φ(H2) is 10%, φ(CO) increases from 30% to 40%, Ri increases at all temperatures, the range over which it rises decreases with increasing temperature, and RC decreases. At 1173 K and 1273 K, because of the low gasification reaction rate of the coke,31) the RC value is also low, and an increase in φ(CO) has little effect on RC. When the temperature rises to 1373 K and 1473 K, RC decreases by 1.37 percentage points and 3.38 percentage points, respectively. This is due to the competition between CO and H2 for iron ore reduction when CO and H2 coexist in the gas. The reduction rate of H2 for iron ore is reduced with an increase in φ(CO), and φ(H2O) decreases relatively, which reduces RC. This is proven by thermodynamic calculations.

Effect of CO on Ri and RC under different conditions. (Online version in color.)
The volume fractions of CO2 and H2O produced by iron ore reduction in different atmospheres are calculated thermodynamically. The volume fractions of CO in atmospheres a, b, c and d increased from 30% to 40% with and without H2, respectively. Figure 7 shows the variation in φ(CO2) and φ(H2O) produced during the reduction of iron oxides in the four atmospheres. As shown in Fig. 7, in the presence or absence of H2 in the gas, increasing φ(CO) increases φ(CO2). The amount of φ(CO2) decreases with the addition of H2, and the range over which it decreases increases with rising temperatures. In the presence of H2, increasing φ(CO) decreases φ(H2O), and φ(CO2)/φ(H2O) increases gradually.

Variation of φ(CO2) and φ(H2O) under different conditions. (Online version in color.)
Figure 8 shows the effect of H2 on iron ore reduction and coke gasification. Figure 8 shows that Ri increases significantly when φ(H2) increases from 0% to 10%. Compared with Figs. 8(a) and 8(b), RC increases with increasing φ(H2), but the range over which it rises decreases with increasing φ(CO), indicating that the effect of H2 on coke decreases with increasing φ(CO) in the blast furnace.

Effect of H2 on Ri and RC under different conditions. (Online version in color.)
To compare the effects of H2 and CO on the synergistic reaction between coke and ore, experiments were carried out in a (40% CO + 0% H2) atmosphere and a (30% CO + 10% H2) atmosphere. The Ri and RC values at different temperatures are shown in Fig. 9. From Fig. 9, it can be seen that under the premise that the concentration of the reducing gas in the gas remains unchanged, 10% H2 is used to replace 10% CO for reduction, and Ri increases at all temperatures. With increasing temperature, the range over which it rises gradually increases, indicating that the reduction ability of H2 is stronger than that of CO. The RC value also increases at different temperatures, and the range over which it rises gradually increases with increasing temperature. This result is because the φ(H2O) produced by the reduction reaction increases after the replacement of CO with H2, and the gasification reaction rate of H2O and coke is much higher than that of CO2 and coke,8) so the RC value increases at all temperatures.

Comparison of the effects of H2 and CO on Ri and RC. (Online version in color.)
Figure 10 shows the reaction rate of iron ore and coke at different temperatures. The reaction rate of iron ore (ri) and the reaction rate of coke gasification (rC) are obtained by Eqs. (15) and (16), respectively.
| (15) |
| (16) |

Reaction rate of coke gasification and ore reduction under different conditions. (Online version in color.)
Figure 10 shows that the reduction rate of iron ore is higher than the gasification rate of coke at all temperatures. In each atmosphere, ri first increases and then decreases with increasing temperature, and rC increases gradually. With increasing the reduction degree of iron ore, the content of the remaining unreduced oxides decreases, and the internal diffusion resistance increases during the reduction process, so ri shows a decreasing trend in the high temperature region.23) The ri in atmosphere b is higher than that in atmosphere a. This makes the amount of CO2 produced in atmosphere b greater than that in atmosphere a, which makes the rC in atmosphere b higher than that in atmosphere a. Under atmosphere c, ri is always higher than that in atmosphere b; that is, the reduction rate of H2 to iron ore is higher than that of CO to iron ore, and because of the production of H2O during the reaction, the rC in atmosphere c is higher than that in atmosphere b. Under atmosphere d, due to the large reduction of iron ore in low temperature region, the volume fraction of CO2 and H2O produces in high temperature region reduces, which limits the increasing trend of rC in high temperature region, and hinders the gasification of coke.
The total amount of CO2 and H2O (ra, mol/min) produced by the reduction of iron oxide and the total amount of CO2 and H2O (rb, mol/min) consumed in the coke gasification reaction can be obtained from Eqs. (17) and (18). η is the percentage of total CO2 and H2O consumed by coke gasification in total CO2 and H2O produced by the reduction of iron oxide.
| (17) |
| (18) |
| (19) |
Under different atmospheric conditions, ra and rb at different temperatures are shown in Fig. 11, and the relationships between η and T are shown in Fig. 12. In all the experimental atmospheres, ra reaches its maximum value at 1273 K, but because of the low gasification reaction rate of coke at this temperature, rb is low, and η is lower than 2%. When the temperature is over 1273 K, ra decreases to different degrees, rb increases significantly with increasing temperature, and η also increases significantly. At 1373 K, η increases to 15.51% and 13.64% in atmospheres c and d, respectively, which are higher than atmospheres a and b. At 1473 K, η in all the experimental atmospheres increases significantly, higher than 40%. When the gas contains 10% H2, the increase in φ(CO) causes ra to increase at 1173 K and 1273 K, while ra decreases at 1373 K and 1473 K; that is, iron ore is greatly reduced in the low-temperature area, reducing the production of CO2 and H2O in the high-temperature area.
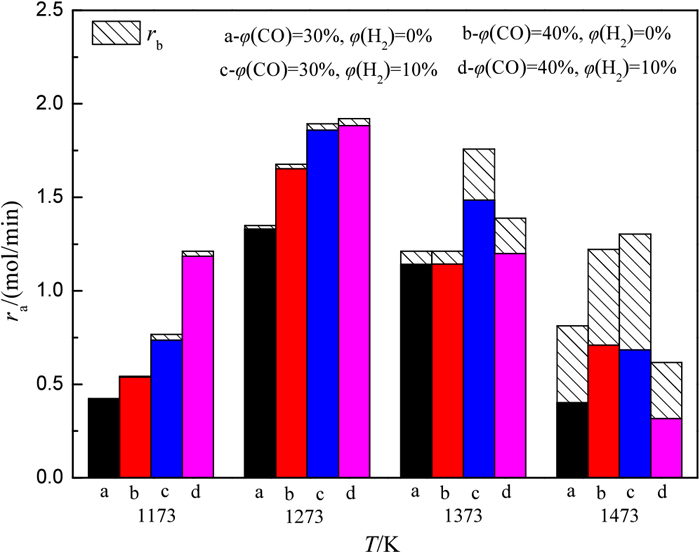
ra and rb at different temperatures. (Online version in color.)

Relationships between η and T. (Online version in color.)
The above analysis shows that increasing the volume fraction of CO in the blast furnace gas without H2 can promote the gasification reaction of coke. Increasing the volume fraction of CO in the blast furnace gas with H2 can restrain the gasification of coke and maintain the high temperature strength of coke. Figure 13 is the schematic diagram of the influence mechanism. In the absence of H2, the total amount of CO2 produced by reduction of sinter and pellets and the consumption of CO2 in coke gasification are less in the low temperature region. With the increase of temperature, the total amount of CO2 produced by reduction of sinter and pellets and the amount of CO2 consumed by coke gasification increase. In the presence of H2, the sinter and pellets are reduced in low temperature region to produce a large amount of CO2 and H2O. The gasification rate of coke increases to a certain extent compared with that without H2. It is mainly due to the presence of H2O in the gas, the gasification reaction of coke with H2O has lower reaction start temperature and higher reaction rate than that with CO2, although the total amount of CO2 and H2O produced by reduction of sinter and pellets in high temperature zone decreases with increasing temperature, the gasification amount of coke still increases greatly due to the presence of H2O. Namely, the reduction rate of sinter and pellets is increased when H2 is added to the gas, the reduction of sinter and pellets in the low temperature zone as much as possible. Although the ra produced in the high temperature zone is reduced, the gasification reaction of coke is still promoted due to the increase of φ(H2O).

Schematic diagram of influence mechanism. (Online version in color.)
In order to further analyze the destructive effect on the pore structure of coke under different atmosphere conditions, the porosity of zone 1 and zone 2 in Fig. 14 are measured respectively. Figure 15 shows the porosity of zone 1 and zone 2 under different conditions. Figure 15 shows that with the increase of temperature, the porosity of zone 1 and zone 2 increases gradually in each atmosphere.

Schematic diagram of coke porosity measurement area. (Online version in color.)

Porosity of coke zone 1 and zone 2 under different conditions. (Online version in color.)
In Fig. 15(a), the porosity difference of each atmosphere at 1173 K is relatively small, which is mainly due to the low gasification reaction rate of coke at this temperature and the low consumption of fixed carbon. The porosity of zone 1 at 1273 K is 42.51%, slightly higher than that of other atmospheres. With continuous increasing temperature, the porosity of zone 1 increases fastest in atmosphere c, followed by that of atmosphere d and atmosphere a. The zone 1 reacts first in the gasification process of coke, and its porosity is directly affected by the fixed carbon consumption. The detection results of porosity in zone 1 are consistent with the gasification reaction rate obtained in the experiments.
In Fig. 15(b), the porosity of zone 2 in atmosphere c and d at 1173 K and 1273 K is slightly higher than that in atmosphere a and b, indicating that the existence of H2 increases the porosity of zone 2 under this condition, which is mainly due to the porosity of zone 2 mainly determined by the diffusion ability of the gas. During the reduction process of H2, H2O is produced, and the initial reaction temperature of H2O and coke is lower than that of CO2,14) and the diffusion ability of H2O is stronger than that of CO2,8) so that under this condition, more reaction gases can pass through the reaction zone 1 and consume the fixed carbon in the zone 2, thus increasing the porosity of the zone 2. With the increase of temperature to 1473 K, the porosity of zone 2 increases slowly in the presence of H2. At 1473 K, the porosity of zone 2 in atmosphere c and d is lower than that of atmosphere b. The main reason is that with the increase of temperature, the gasification reaction rate of coke increases significantly. Compared with CO2, the gasification reaction of H2O and coke takes place more in zone 1, and the amount of gas that can diffuse to zone 2 is reduced, which makes the porosity of zone 1 increase more greatly, while that of zone 2 is relatively small. Wang et al.14) studied the influence of pure H2O and pure CO2 on the gasification process of coke, it was pointed out that the reaction of coke and H2O occurred more on the surface of coke than that of coke and CO2 at high temperature, which made the damage of H2O to coke weaker than that of CO2, but the destruction effect of H2O on coke was stronger than that of CO2 at low temperature. This conclusion is consistent with the research results of this paper, which can explain the research results of this paper well. The above analysis shows that the presence of H2 will aggravate the increase of coke porosity in the low temperature region, but it reduces the internal porosity in the high temperature region, which will help to improve the strength of coke after high temperature reaction.
With the decrease in φ(CO)/φ(H2), the start and end temperatures of C gasification in the system decrease gradually, and the temperature range of C gasification decreases and moves to the low-temperature zone.
In the absence of H2, Ri and RC increase gradually with increasing φ(CO). In the presence of H2, φ(CO) increases, Ri increases at all temperatures, and RC decreases. Increasing φ(CO) can reduce the effect of H2 on coke. The effect of H2 on coke gasification is stronger than that of CO.
With the increase of φ(CO) and φ(H2), the reduction of iron oxide tends to be carried out in the low-temperature zone, and the φ(CO2) and φ(H2O) produced in the high-temperature zone decreases. The presence of H2 in the gas intensifies the gasification of coke.
The presence of H2 aggravates the increase of coke porosity in the low temperature region, but it reduces the internal porosity in the high temperature region, which will help to improve the strength of coke after high temperature reaction.
The authors are grateful for financial support from the Key Program of the National Natural Science Foundation of China (U1360205, 51674122) and Natural Science Foundation of Hebei Province (E2019209424).