2021 年 61 巻 4 号 p. 1194-1200
2021 年 61 巻 4 号 p. 1194-1200
Wet/dry cyclic corrosion tests were conducted to investigate the effect of relative humidity (RH) at dry periods on the hydrogen permeation behavior of steel. Steel specimens were exposed to a temperature and humidity-controlled environment with an initial placement of NaCl droplet. The RH at the dry period was controlled at 20%, 55%, 60%, and 65%, which was lower than the deliquescence RH of NaCl (75%). Hydrogen permeation current was detected using electrochemical methods. The corrosion process on the steel was observed using optical techniques. The results show that the amount of permeated hydrogen through the steel increases with increasing RH at the dry period from 20% to 65%. The hydrogen evolution reaction is inhibited with the accumulation of corrosion products on the steel surface, resulting in a decrease in the amount of permeated hydrogen. Based on the results, the state of NaCl inside corrosion products is supposed to be a solid phase at 20% RH, a liquid-solid phase at 55% RH, and a liquid-rich phase above 60% RH. The increasing volume of the liquid phase contributes to the rise in the amount of permeated hydrogen at the dry period.
Nowadays high strength steels are widely used for the automobile to achieve weight reduction and meet the increasing demands on fuel efficiency.1) The presence of hydrogen in steels often causes a deleterious effect known as hydrogen embrittlement which limits the further strengthening of steels.2,3,4) Therefore, several studies on investigating the interactions of hydrogen with steels were conducted to understand the mechanism of hydrogen embrittlement.5,6,7,8)
Atmospheric corrosion is the most prevalent type of corrosion for steels and is considered to be an important source of diffusible hydrogen.9,10,11,12) When steels are used under atmospheric conditions, hygroscopic salts such as NaCl and MgCl2 tend to form thin electrolyte films on the steel surface when a critical relative humidity level is reached.10,13) In the presence of thin electrolyte films, electrochemical corrosion processes are carried out and hydrogen evolution reactions take place. The hydrogen atoms generated from corrosion can be absorbed into the steel and diffuse into the region where crack potentially occurs, which causes the fracture of steels. Therefore, it is required to clarify the behavior of hydrogen permeation into steel during chloride salts induced atmospheric corrosion.
Extensive research results showed that the hydrogen absorption process underneath the chloride contained solution layers on the steel surface was affected by many environmental factors, such as temperature, humidity, and the concentration of electrolyte.14,15,16) Omura et al.17) investigated the influence of relative humidity (RH) on hydrogen entry into steel by the sea salts containing NaCl and MgCl2, and reported that hydrogen permeation coefficients were higher at 40–60% RH. This result was explained by the increase in the concentration of Cl− with the decreasing solution layer thickness. The high concentration of Cl− at the anode site is known to accelerate the oxidation of dissolved iron(II) ions and hydrolysis of formed iron(III).18,19) Haruna et al.20) reported that the hydrogen absorption behavior of Fe under the rust layer containing NaCl was affected by the RH and supposed that some NaCl droplets were formed in the rust layer when the RH is lower than the critical RH of NaCl. The effect of RH on the formation of the electrolyte layer on the steel surface is generally considered as an important factor in corrosion reaction with hydrogen evolution.
The droplet on the steel surface usually contains several mixed chloride salts with different solubility.10) When the RH reaches a specific value known as deliquescence relative humidity (DRH), the chloride salts will absorb water to form aqueous electrolyte layers.21,22,23) For example, a critical RH value (75%) is required for NaCl particles deposited on the steel surface to deliquesce and form solution layers where considerable corrosion will take place. Schindelholz et al.24) examined the corrosion of steel below the DRH of NaCl and found that the corrosion could initiate at 33% RH. They also proposed that the RH at least 40–50% might be sufficient for the formation of conductive electrolyte on NaCl crystals. Hence, the state of salts on the steel surface is expected to be a crucial factor for the hydrogen evolution during corrosion reactions below the deliquescence point of chloride salts. However, the behavior of hydrogen permeation through the steel under controlled RH below the deliquescence value of chloride salts during the corrosion process is not fully understood.
In the present study, the NaCl solution was used for hydrogen permeation tests under controlled RH below the DRH of NaCl at dry periods during cyclic wet/dry processes. The purpose of this study is to investigate the effect of RH at the dry period on the hydrogen permeation behavior of steel during the chloride-induced wet/dry corrosion.
The material used in this study was Japanese industrial standards (JIS) SCM 435 steel with the chemical composition listed in Table 1. Specimens with the dimensions of 15 mm × 20 mm were cut from 1 mm thick plates and then were mounted in cold-curing resin with one of the faces exposed at the bottom of the mount for polishing. Both sides of the specimens were mechanically polished with SiC grinding papers up to 1500 grit. The polished specimens were removed from the resin and were ultrasonically cleaned with ethanol and highly purified water. After ultrasonic cleaning, one side of the specimen was used as an exposure surface for hydrogen absorption. The other side of the specimen was used for hydrogen detection and was electroplated in a 0.312 kmol m−3 NiSO4/0.781 kmol m−3 H3BO3 solution at 4 mA cm−2 for 720 s to form a Ni layer with a thickness of about 1 μm. The specimens were cleaned with ethanol and highly purified water in an ultrasonic bath for 300 s before hydrogen permeation tests. 10 mol m−3 NaCl was used as the test solution.
| C | Si | Mn | P | S | Cr | Mo |
|---|---|---|---|---|---|---|
| 0.35 | 0.18 | 0.74 | 0.0011 | 0.0017 | 1.15 | 0.15 |
Based on the Devanathan-Stachurski25) method, an electrochemical cell as shown in Fig. 1 was used to measure the hydrogen permeation current (ihp). Pt wires (diameter: 0.5 mm) were used as the counter electrode (CE) and the reference electrode (RE) of the micro-electrochemical cell. The Ni-plated side of the specimen was attached to the cylindrical electrochemical cell (diameter: 6 mm) filled with 1 kmol m−3 NaOH solution. The opposite side was exposed to the air as the position of the droplet. The electrochemical cell with the specimen was set in an RH and temperature-controlled chamber as shown in Fig. 2. The current was detected and recorded by a data logger connected to the potentiostat. Before the wet/dry corrosion tests, the specimen was polarized at −30 mV vs. Pt by using the potentiostat. After the background current reached lower than 40 nA cm−2, 10 μL test solution was placed on the specimen surface to initiate the corrosion process. In addition, the background current that varied with temperature was recorded separately and then was subtracted from the detected current in wet/dry corrosion tests to obtain accurate permeation current.

Schematic drawing of the electrochemical cell.

Schematic drawing of the experimental system.
Wet/dry cyclic corrosion tests were carried out according to four stages (wet period, drying period, dry period, and wetting period) in one cycle as shown in Fig. 3. The minimum RH value during the cyclic wet/dry process was set at 20%, 55%, 60%, and 65%. The RH values at the dry period were lower than the DRH of NaCl. Initially, the temperature and RH were controlled at 303 K and 90% for 1 h. After the wet period, the temperature increased to reach 323 K while the RH decreased to the set value. The RH at the dry period was kept at the set value, and the temperature was constant at 323 K in the chamber. After the dry period (3 h), the temperature decreased to 303 K, and the RH increased to 90%. The corrosion process was carried out for 12 cycles (72 h).

Schematic representation of the changes in relative humidity (RH) and temperature during one cycle of the wet/dry corrosion tests. (Online version in color.)
To understand the corrosion process of steel under cyclic wet/dry conditions, an endoscope was fixed above the exposed surface of the specimen to observe the corroded region during the tests (Fig. 2). An optical microscope was used to characterize the corroded surface of the specimen after the wet/dry corrosion test.
Figure 4 shows the changes in ihp of specimens under different RH values at the dry period during wet and dry cyclic corrosion tests for 12 cycles (72 h). At the initial stage, the ihp increases gradually and then tends to keep steady when the temperature is constant at 303 K (Fig. 3). This result suggests that the corrosion reaction with hydrogen evolution was initiated after the solution was placed on the steel surface. After the wet period (1 h), the ihp increases at a rapid rate to reach a peak value with the gradual increase of temperature. When the RH values of the dry period are controlled higher than 60%, the peak values of the ihp are larger than the other conditions. The drastic increase in ihp is attributed to the acceleration of hydrogen evolution reaction and hydrogen diffusion, which can be accelerated by the increasing temperature. The ihp, except the condition under 55% RH at the dry period, decreases swiftly at the early stage of the dry stage. When the RH at the dry period is controlled higher than 55%, the ihp keeps at a larger value compared with the wet period. This result indicates that the hydrogen evolution reaction occurs on the steel surface even though the RH is lower than the DRH of NaCl. After the dry period (3 h), the ihp shows a rapid decrease at the wetting period due to the decreasing temperature.

Changes in permeation current of specimens during wet/dry cyclic corrosion tests under different RH values at the dry period. (Online version in color.)
After the second cycle, the peak value at the beginning of the dry period decreases with the increase of cycle number. Some humps are observed on the shoulder of the ihp at the dry period, which indicates that the hydrogen evolution reaction is accelerated in these periods. In addition, the ihp at dry periods became steady after several cycles. The ihp under 20% RH becomes steady at the earliest after 2 cycles, while the ihp under RH higher than 60% takes a long time (about 9 cycles) to drive at a steady value at the dry period. The changes in ihp of each cycle are comparable after the ihp keeps at a steady state.
Figure 5 shows the changes in ihp during the 10th cycle of wet/dry corrosion tests. The ihp varies in a lower range at the wet period and then increases at different rates, contrary to the rates at which RH decreases. The ihp values at the dry period of each cycle show a distinct disparity between different RH values and increases with increasing RH. The ihp at the wet period is always lower than the dry period. The ihp increases and decreases drastically at the drying period and the wetting period. These results indicate that temperature and RH play an important role in the hydrogen evolution reaction during wet/dry corrosion processes. In addition, the ihp decreases gradually at the dry period when the RH is higher than 55%, whereas at 20% RH, the ihp shows a slight increase.

Changes in permeation current of specimens during the 10th cycle. (Online version in color.)
The area under the current curve in each cycle represents the electric charge, which is related to the amount of permeated hydrogen. Figure 6(a) shows the electric charge at the dry period of each cycle as a function of cycle number. The electric charge of each cycle decreases with the increase of the cycle number and tends to keep steady after several cycles. The difference between the electric charges under different RH conditions is not statistically significant during the initial cycles. After the electric charges become steady, there is a significant difference in the charges under controlled RH values. The electric charge of each cycle increases with increasing RH at the dry period. Figure 6(b) reflects the total electric charge during the wet/dry cyclic corrosion test as a function of RH value at the dry period. The hydrogen permeation charge increases with increasing RH. The electric charge is comparable when the RH of dry periods is higher than 60%. These results indicate that the increasing RH at dry periods accelerates the hydrogen evolution reaction.

(a) The electric charge at the dry period of each cycle as a function of cycle number. (b) The total electric charge of each wet/dry corrosion test as a function of RH value at the dry period. The error bars indicate 95% confidence intervals. (Online version in color.)
To understand the relationship between the corrosion process and the hydrogen permeation behavior, the optical endoscope images of the corrosion process on the specimen surface during cyclic corrosion tests were obtained as shown in Fig. 7. Initially, 10 mol m−3 NaCl solution is placed on the specimen surface and no corrosion products are observed under the droplet (Fig. 7(a)). At the wet period of the first cycle (T: 303 K, RH: 90%), some orange corrosion products are generated on the steel surface under NaCl droplet after 30 min (Fig. 7(b)). This means the corrosion reaction with hydrogen evolution initiates under the droplet combined with the changes in ihp as shown in Fig. 4. The orange corrosion products flowed and were scattered in the NaCl solution. At the latter stage of the wet period, the amount of orange corrosion product has spread over the entire droplet area (Fig. 7(c)). After the wet period (1 h), the increase in temperature and the decrease in RH accelerate the evaporation of water and promote the formation of corrosion products. This is considered to enhance the hydrogen evolution and result in the rapid increase of the ihp at the drying period. With the formation of more corrosion defects, more orange rust accumulates on the specimen surface along the perimeter of the droplet to form a perceptible rust ring (Fig. 7(d)). The corrosion products spread out adjacent to the rust. These results indicate the corrosion reaction is conducted at the drying period. A thin film of corrosion products on the surface and a portion of brown rust are observed at the later stage of the drying period (Fig. 7(e)). The specimen is covered with rust at the dry period. A small increase in the area of black rust and no big changes in the amount of corrosion products are observed using the endoscope (Fig. 7(f)). This result indicates that the corrosion reaction proceeds slowly at the dry period. With the increase in RH at the wetting period, the surface of corrosion products begins to be moist. A small amount of corrosion products spreads out nearby the iron rust produced at the previous stages (Fig. 7(g)). The ihp shows a rapid decrease at the wetting period even though the corrosion reaction is carried out on the steel surface. This result indicates that the decrease in the hydrogen evolution on the surface and the diffusion in steels are attributed to decreasing temperature. The amount of corrosion product shows a gradual increase on the steel surface during the 12 cycles (Figs. 7(h)–7(n)). The results show that an amount of black iron rust accumulates along the perimeter of the rust circle and a portion of rust is generated near the boundary of the previous rust.

Time sequenced endoscope images of the specimen under 55% RH at the dry period during wet/dry cyclic corrosion tests. (Online version in color.)
Figure 8 shows the optical microscope images of corroded surfaces of specimens after 12 wet/dry cycles. When the RH at the dry period is controlled above 60%, the amount of corrosion products is larger than the conditions under a lower RH. This result indicates that the amount of permeated hydrogen is related to the corrosion reaction. A notable orange rust ring can be observed on each specimen surface. When the RH at the dry period is higher than 55%, a large amount of black iron rust accumulates in the center and perimeter of the ring.
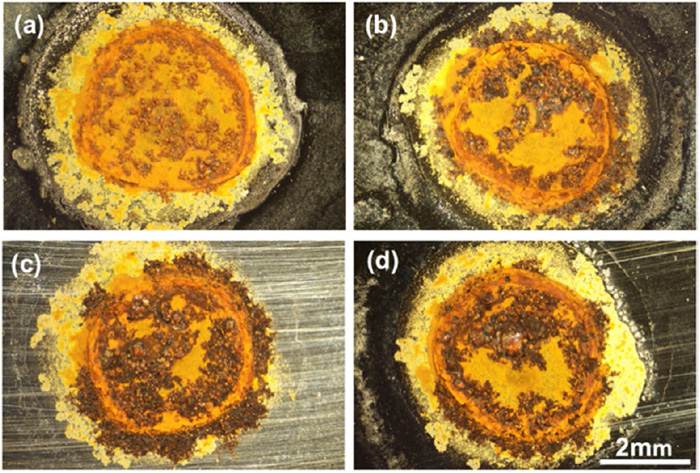
Optical microscope images of corroded surface after wet/dry cyclic corrosion tests with different RH values (a) 20%, (b) 55%, (c) 60% and (d) 65% at the dry period. (Online version in color.)
This study examined the changes in the hydrogen permeation current under different RH values at dry periods during wet/dry corrosion tests. The results suggest that the hydrogen permeation behavior of the steel is associated with the corrosion reaction that is affected by the RH.
4.1. Mechanism of Hydrogen Evolution Reaction on the Steel SurfaceAt the initial stage, electrochemical corrosion processes are carried out on the steel surface due to the presence of the droplet containing Cl− and proceed by balancing anodic and cathodic reactions as shown in Fig. 9. The area adjacent to the edge of the droplet provides easy access for oxygen to reach the steel surface and plays the role of the cathode with the reaction of oxygen reduction (Eq. (1)). The Fe2+ generated at the anodic site migrates into the cathodic site to form iron rust with OH− generated by the oxygen reduction reaction (Eq. (2)). In addition, the Fe2+ derived from Fe(OH)2 will be oxidized to Fe(OH)3 by the dissolved oxygen (Eq. (3)).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |

Schematic representation of the steel corrosion process with hydrogen evolution under a droplet. (Online version in color.)
At the drying period, the ihp shows a rapid increase with the increasing temperature because the temperature has a positive influence on the hydrogen evolution reaction and diffusivity in the steel.26) Moreover, the concentration of Cl− contained in the solution layer increases as the evaporation of water on the steel surface, which is considered to accelerate the corrosion reactions and the evolution of hydrogen. Thus, the rapid increase in ihp during the drying period is also attributed to the increasing concentration of Cl−. After the drying period, temperature and RH are constant. The corrosion reaction with hydrogen evolution is inhibited due to the shrinking volume of the solution layer on the steel surface. The ihp, therefore, begins to decrease at the dry period. This is the reason why the peaks can be observed at the beginning of dry periods (Fig. 4).
The presence of NaCl particles in the rust layers plays a crucial role in the corrosion process at the dry period. NaCl particles possibly are moist and form thin electrolyte layers at the interface between rust and steel substrate, considering that the capillary cohesion of water molecules may be increased by the corrosion products.24,27) Figure 10 shows the schematic representation of the corrosion processes at the period of lower RH than DRH of NaCl. When the RH is kept higher than 55%, a portion of NaCl particles may deliquesce to form thin electrolyte layers under the rust. The electrolyte anions Cl− migrate to the anodic site and balance the positive charge of dissolved Fe2+. The electrolyte cations Na+ migrate to the cathodic site and balance the negative charge of OH−. Electric neutrality is maintained, and corrosion reaction proceeds at the dry period.

Schematic representation of the corrosion process at the dry period under (a) 20%, (b) 55% RH, (c) 60% RH and (d) 65% RH. (Online version in color.)
At the dry period, the rust on the steel surface is considered to be dehydrated due to the low RH, resulting in cracks in the rust layer. These cracks provide the routes for oxygen to reach the steel surface. Thus, the Fe2+ formed in the anode site can be oxidized by the oxygen (Eq. (7)). The accumulation of H+ generated in the anodic site (Eqs. (6), (7)) enhances the hydrogen evolution reaction and contributes to the increase in the ihp at dry periods in some cycles. As the corrosion reaction proceeds, the generated corrosion products are expected to block the entry of oxygen as well as the migration of ions between the cathodic and anodic sites, the ihp decreases due to the suppression of the hydrogen evolution and corrosion reaction. These phenomena on ihp are represented as the hump shape on the shoulder of the current curve at the dry period (Fig. 4).
4.3. The Effect of Different RH on the Hydrogen Permeation Behavior at the Dry PeriodThe distinct differences in ihp under controlled RH from 20% to 65% are supposed to be due to the changes in the volume of dissolved NaCl inside the rust layer, as shown in Fig. 10. When the RH is controlled at 20%, the NaCl particle is difficult to absorb water and deliquesce into a liquid phase. There is almost no available electrolyte for the corrosion reaction on the steel surface. When the RH is above 55%, the significant increase in the ihp at some dry periods suggests that there may be deliquesced NaCl particles inside the corrosion product. The state of the NaCl particle inside the corrosion product is supposed to be a solid-liquid phase at 55% RH. The volume of the solution layer increases with increasing RH, and more cathodic areas can be connected to enhance the corrosion reaction as well as the hydrogen evolution reaction. After several cycles, the corrosion reactions are inhibited, and the ihp tends to keep at a steady decrease at the dry period when the RH above 55%. It is a possible reason that the migration of ions between anodic and cathodic sites is obstructed, which is attributed to the accumulation of corrosion products. In the case of 20% RH, the amount of the corrosion product on the steel is smaller than the other three conditions, which causes more oxygen to pass through the corrosion product and reach the steel surface for the corrosion reaction. As the corrosion reaction proceeds, the hydrogen evolution reaction can be accelerated by the gradual accumulation of Cl− in the corrosion area, resulting in a slight increase in the ihp at the dry period.
In this study, steel with an initial deposition of NaCl droplet was used to investigate the hydrogen permeation behavior under different RH controlled below the DRH of NaCl at dry periods. The effect of RH on hydrogen permeation behavior of steels was studied by hydrogen permeation tests under wet/dry corrosion environments. Optical techniques were used to understand the corrosion process of steels under the electrolyte layer. Based on the results, the following conclusions could be drawn:
(1) Corrosion reaction with hydrogen evolution can occur on the steel surface even the RH is below the DRH of NaCl and is inhibited with the accumulation of corrosion products and the decreasing RH from 65% to 20%.
(2) The amount of permeated hydrogen at the dry period of the wet/dry corrosion process increases with increasing RH from 20% to 65%, which is attributed to the increasing volume of liquid phase inside the corrosion product.
(3) The changes in permeation current can be explained in terms of the state of NaCl inside the corrosion product. The NaCl particle is supposed to be a solid phase at 20% RH, a liquid-solid phase at 55% RH, and a liquid-rich phase above 60% RH.
The authors acknowledge the research groups, “Comprehensive Understanding of Hydrogen-Passive Surface on Steels for Prevention of Hydrogen Embrittlement” and “Corrosion-induced Hydrogen Absorption to Steels” of the Iron and Steel Institute of Japan, for their sufficient contribution in the discussion of our research results as well as their financial support.