2021 Volume 61 Issue 5 Pages 1539-1549
2021 Volume 61 Issue 5 Pages 1539-1549
Several X-ray topography studies which have appeared recently in the literature show compacted graphite in cast iron consisting of coarse interconnected graphite lamellas. This suggested that solidification of these alloys could be described as done for irregular eutectics by accounting for limited branching of graphite lamellas. The corresponding growth law has been inserted in appropriate mass balances for describing the successive solidification stages. Predictions of the model thus obtained have been compared to quantitative experimental information previously gained on solidification of a series of hyper-eutectic alloys. The deep undercooling and marked recalescence which are so characteristic of the solidification of compacted graphite cast irons in the stable system are reproduced and appear to be closely related to the limited branching of graphite lamellas in the compacted graphite cells. The competition between stable and metastable solidification could be described and leads to a decrease of the recalescence amplitude that was properly reproduced.
Thanks to detailed metallographic studies1,2) and to recent tomography results,3,4) it is now well established that graphite in compacted graphite (CG) cells consists of interconnected thick lamellas with bumpy surfaces as illustrated in Fig. 1. Because of the appearance of CG as worm-like particles in 2D sections with sometimes hemispherical caps, many early studies5,6) concluded that the overall growth direction should be the basal plane direction as in spheroidal graphite. This however appears contradictory with the lamellar growth shape shown with recent tomographic results. A detailed study combining observations with polarized light microscopy and scanning electron microscopy (SEM) of deep etched and ion etched samples led Franklin to the conclusion that the main growth direction of compacted graphite is parallel to the basal plane of graphite,7) i.e. along the prismatic direction. This was further confirmed more recently by Holmgren et al.8) who observed by electron backscattering diffractometry (EBSD) that worm-like graphite particles are oriented along the prismatic direction as lamellar graphite while protuberances seem to develop in the basal direction as in spheroidal graphite. Accepting that growth of CG cells proceeds mainly as those in lamellar graphite irons, it has been proposed that the main difference between lamellar and compacted graphite structure is the very limited branching of the lamellas in compacted graphite iron (CGI).9,10)

SEM view a CG cell after deep etching of the material to remove the matrix (courtesy of W. Guesser).
It seems also agreed that the CG cells develop around previously precipitated graphite spheroids11,12) as a kind of graphite degeneracy.6) In the case of hypereutectic alloys, the observation of large primary graphite spheroids sustains this claim. The same schematic has been accepted by Lopez et al.13) for hypoeutectic alloys. It is not unusual to observe also small nodules in CGI which formed at the end of solidification; they have been associated to a combination of large undercooling and Mg micro-segregation during bulk eutectic solidification.14,15)
Based on the above view of CGI solidification, a physical model of solidification of CGI is described for the case of hypereutectic composition. This model is then applied to experimental results on non-inoculated alloys which have been detailed previously16,17) and relate to solidification in small thermal analysis cups.
Figure 2 shows the isopleth Fe–C section at 3.65 mass% C, 0.83 mass% Cu, 0.62 mass% Mn and 2.39 mass% Si, that is representative of the final composition of the experimental alloys. The data used to draw this section have been detailed previously17) and are summarized in appendix A. This isopleth section shows the stable diagram in solid lines with their metastable extrapolation drawn with interrupted lines. The intersection between the two liquidus defines the stable eutectic, Liquid→austenite+graphite at the temperature, TEUT, shown with the upper horizontal dashed line. The austenite solidus was calculated assuming a constant carbon partition coefficient

In bold lines is shown the isopleth Fe–C section of the Fe–C–Si–Mn–Cu phase diagram at 0.83 mass% Cu, 0.62 mass% Mn and 2.39 mass% Si; the interrupted parts of the lines are metastable extrapolations. The thin horizontal dashed lines indicate the temperature of the stable, TEUT, and metastable, TEW, eutectics in this isopleth section. Thin solid lines represent schematically the solidification path of a hypereutectic alloy and the thin horizontal doted lines show the corresponding characteristic temperatures,
In all the following, the alloys will be considered as pseudo binary Fe–C alloys and their solidification will be described in the isopleth section such as that shown in Fig. 2. On this figure, the solidification path of a hypereutectic alloy is represented with thin solid lines along 3 successive steps labelled a, b and c, that are described as follows. When the temperature is decreased from the full liquid state, the solidification path reaches the graphite liquidus at

Schematic illustrating the three successive steps in the solidification of an hypereutectic CGI as labelled in Fig. 2: a) precipitation of primary graphite; b) growth of off-eutectic austenite dendrites and of CG cells; c) additional formation of ledeburite cells. (Online version in color.)
When the metastable extrapolation of the austenite liquidus is reached at TLA, formation of austenite dendrites and CG cells may proceed, see Fig. 3(b). It is assumed that equilibrium at the liquid/austenite interface is achieved at any time, meaning that there is no nucleation barrier for austenite and that the composition of the remaining liquid follows the austenite liquidus as indicated with the label b in Fig. 2. The experimental results17) suggest that there is little or no further nucleation of graphite during this stage, so that the number of CG cells could be fixed as the nodule count reached at the end of primary deposition. The equations describing the eutectic reaction in appendix C have been derived for single sized primary graphite, i.e. an equivalent radius RG,eq has been calculated. This is not expected to affect much the calculations as compared to those which could have been made with a size distribution of the primary graphite precipitates, and this makes the equations much lighter.
Finally, if the temperature falls below the temperature of the metastable eutectic, TEW, nucleation of ledeburite is assumed instantaneous. During further solidification, growth of CG cells and ledeburite proceeds (see Fig. 3(c)) while the solidification path still follows the austenite liquidus as indicated with label c in Fig. 2.
While appendices B and C detail the overall kinetics equations, it is worth considering in this section the way growth of the CG cells is to be quantitatively described. According to the introductory part, growth of CG cells is similar to that of irregular eutectics, e.g. lamellar graphite eutectic. The peculiarities of these eutectics have been described in the early 1980s by Fisher and Kurz19) and more particularly by Jones and Kurz20) for the case of the austenite-graphite system. The authors based their analysis on the classical work by Jackson and Hunt21) who stated the following relationship between eutectic undercooling, ΔT, lamellar spacing, λ, and growth rate, Vgrowth:
| (1) |
In Fig. 4, the solid curves represent relation (1) for three growth rates Vgrowth (0.1, 1 and 10 μm·s−1) using the values of a and b estimated by Jones and Kurz:20) a=2.3 μm·K and b=0.080 K·s·μm−2. By stating that growth proceeds at the minimum undercooling one gets the following relationships:
| (2) |
| (3) |
| (4) |

The solid lines show the theoretical evolution of the eutectic undercooling with interlamellar spacing for solidification rates indicated on the curves. The dashed lines show the position of the operative point for two values of φ, namely 1 which corresponds to the minimum undercooling and 10 which corresponds to the branching limit.
Experimental results on growth of lamellar graphite eutectic show that the spacing λ is distributed over quite a large range.20) The boundaries of this range were set to λ0 for the smallest value and λbr for the largest one. λbr is the value at which branching of graphite lamellas must occur for two-phase growth to be maintained. Because of the faceted nature of graphite, branching is quite difficult, and this is thought to explain why λbr has been found to be as large as about 10 times λ0 in lamellar graphite eutectic.20) Assuming the diffusional analysis of Jackson and Hunt remains valid, Jones and Kurz suggested defining an operating interlamellar spacing, λop, such as:
| (5) |
By substitution of λop in Eq. (1) and looking for its extremum (i.e., the minimum undercooling at given Vgrowth), one gets the following expressions for the operating undercooling ΔTop:
| (6) |
| (7) |
This latter relation has been plotted with interrupted lines in Fig. 4 for φ=1 which corresponds to the extremum and φ=10 that relates to the branching limit of graphite lamellas. Following this line, the growth rate of spherical compacted graphite cells of radius RCG will be related to the eutectic undercooling by the following relation where a and b are the values assessed by Jones and Kurz:20)
| (8) |
Jones and Kurz20) could fit their results of directional solidification experiments with φ set to 3.9 while Zou Jie22) found a value of 6.5 for equiaxed solidification of an Fe–C–Si alloy. Zou Jie suggested that part of the difference with directional solidification results is due to the expanding nature of the eutectic cells in equiaxed solidification. As stressed above in the case of CG cells, the protuberances that form on the primary graphite precipitates then develop without much branching. Thus, the distance between them increases as the size of the CG cells increases and should thus reach quickly a high value. This suggested setting φ to the maximum value which should be of the order of 10 where branching of graphite lamellas is expected.
Finally, growth of ledeburite is to be described as spherical cells of radius RW using the data from Hillert and Subba Rao23) which is very close to the value later found by Jones and Kurz:20)
| (9) |
With Eqs. (8) and (9), the change in volume of solid
| (10) |
| V/A [m] | Cp [J·K−1·kg−1] | ρ [kg·m−3] | K [m·s−1] | ΔH [J·kg−1] | |
|---|---|---|---|---|---|
| 0.009 | 728 before solidification 1215 after solidification | Liquid: 920 Solid: 750 | Liquid: 6800 Austenite: 7000 Graphite: 2200 | 0.05 | Graphite: 1.62·106 Iron: 2.56·105 |
Following previous works, the exponent n of the nucleation law for primary precipitation of graphite (see appendix B) was set to 1 so that the nucleation constant is denoted as A1 in the following. Also, it was assumed that a given number of ledeburite cells nucleated as soon as the TEW temperature was reached. The outputs of the calculations are marginally sensitive to the exact value of this number which was set to 0.5 mm−3.
In the following sections, all microstructure parameters keep the same name as in appendices B-D but they are now for a unit volume. This means that NV is a count density and that VS, VCG and Vγ,off are volume fractions.
The experiments considered here have been fully described previously17) and are only shortly presented below. A 4 ton melt prepared for casting spheroidal graphite cast iron was maintained for 8 hours under nitrogen so that the spheroidizing treatment faded slowly leading to precipitation of compacted graphite. Every 20–25 min, two thermal analysis (TA) cups and a medal for chemical analysis were cast; 19 castings were thus carried out which were labelled A to S. The cavity of the thermal cups are truncated pyramids with the small base of 32×32 mm2, the large base of 37×37 mm2 and the height of 40 mm. At the time of pouring, one cup was empty while the other contained an inoculant powder, so that the two samples cast at a given holding time were denoted Xinoc and Xno-inoc, respectively, where X is a letter from A to S. Chemical analysis showed the alloys lose only up to about 0.1 mass% carbon and 0.06 mass% silicon during the 8 h holding, but also a much higher proportion of spheroidizing elements Mg, Ce and La. The cooling curves of the inoculated alloys consisted in one single plateau which did not show much change with holding time although the microstructure changed from fully spheroidal to nearly fully compacted. In strong contrast, the cooling curves for the not-inoculated alloys showed a minimum temperature that decreased continuously with holding time, being below TEW after less than 1 hour. The graphite changed from spheroidal to compacted while more and more carbides were observed. Records from non-inoculated castings did thus contain much more information than those from the inoculated ones and were accordingly selected for the present work as they are more challenging for checking the validity of the modelling approach.
For performing the calculations for the whole series of alloys, it was intended to have as few parameters as possible. Preliminary calculations dedicated to fit the cooling rates before and after solidification led to select a modulus of 0.009 m and a value of
Focus was then first put on the number of graphite spheroids nucleated during primary precipitation of graphite. According to the assumptions indicated above, this number equals the CG cells count that will develop during the eutectic reaction. Figure 5 shows the evolution of the experimental cell count as evaluated on 2D sections, NA, and then converted to 3D values, NV, by
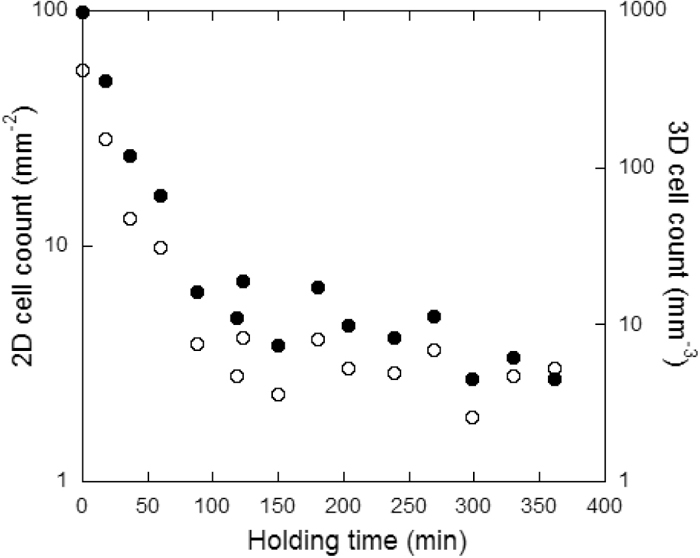
Evolution of the 2D (solid symbols) and 3D (open symbols) experimental cell counts with holding time of the melt.
As an example, Fig. 6 compares the evolution of the calculated and experimental temperatures for alloy Cno-inoc with A1=0.5 mm−3·K−1. It is seen an overall good agreement between the calculated and experimental cooling curves with however a time shift of the experimental curve at the start of eutectic solidification which is not reproduced by the calculation and will be discussed further below. Also shown in Fig. 6 are the predicted overall solid fraction, VS, the amount of off-eutectic austenite, Vγ,off, and that of CG eutectic, VCG. Primary precipitation of graphite is not plotted because the fraction was always very low at less than 1%. It is seen that this is mainly austenite that deposits at the start of solidification. The amount of CG eutectic remains very low until the temperature falls down to just above 1120°C when bulk eutectic sets up with recalescence due to rapid growth of eutectic cells, while part of the off-eutectic austenite remelts before growing again. It is noteworthy that the value of φ=10 that was selected leads to a fairly good description of the recalescence amplitude. A lower value of φ would relate to a lower growth undercooling (see Fig. 4) and thus to both a higher minimum temperature and a lower recalescence amplitude.

Experimental (dotted curve) and calculated (solid curve) cooling curves for alloy Cno-inoc. Also shown are the predicted overall solid fraction, VS, the amount of off-eutectic austenite, Vγ,off, and that of CG eutectic, VCG. The open arrow indicates the TLA value which corresponds to the temperature when the calculated primary solidification path reaches the extrapolation of the austenite liquidus. (Online version in color.)
In the calculations as the one shown in Fig. 6, formation of austenite at the end of primary precipitation of graphite leads to a slope change at the TLA temperature that is indicated with the open arrow in the figure. In contrast, all experimental cooling curves for not inoculated alloys B-S showed a slope change soon followed by a short plateau similar to that seen in Fig. 6. Further, calculation for the whole series of alloys made it evident that the experimental TLA value was lower than the calculated one. This is illustrated in Fig. 7 where it is seen that the experimental values vary in between 1138 and 1153°C and that they are 5 to 10°C lower than the calculated values. The change in the experimental value of TLA was observed to be an overall increase with holding time.17) As a matter of fact, the TLA value is expected to increase when: i) the pouring temperature is increased leading to lower cooling rate; ii) the carbon equivalent of the alloy is decreased; and iii) the graphite nucleation rate is increased. In fact, most of the change in the experimental TLA value could be accounted for by the decrease in carbon content of the alloys with holding time as discussed previously.17) Change in the pouring temperature could account for a smaller part of the variation, and dedicated calculations (not shown here) demonstrated that the effect of graphite nucleation is negligible for the range of A1 values used here. Summing up, the good linear correlation seen in Fig. 7 demonstrates that the model for primary precipitation of graphite accounts properly of the change in composition and pouring temperature of the alloys, though not describing the systematic undercooling for austenite formation which amounts to 5–10°C. It is noteworthy that this undercooling is well within the range of values discussed by Heine.27)

Experimental versus calculated TLA values. The solid line is linear fit to the data while the interrupted line is the bisector.
Owing to the small size of the thermal cup, it is expected that austenite develops from the outer surface of the cup towards the center as described by Mampaey by means of quenching experiments of rods of similar size as the TA cups.28,29) For such a columnar growth, the thermal arrest when the solidification front reaches the thermocouple is not expected to give rise to recalescence, and recalescence was effectively not observed in the present series of records. In turn, this means that the 5–10°C temperature difference is due to the kinetic undercooling of the front of austenite dendrites. The growth rate
It is expected that a 3D micro-macro modelling of the solidification of the thermal cups would allow recovering the feature described above. For the present approach, it was decided to amend the model by introducing an undercooling of the liquid amounting to ΔTtip before austenite appears in the central area of the TA cups. The solidification path was then changed as illustrated in Fig. 8 with austenite starting to form only when the temperature (TLA-ΔTtip) has been reached. At that temperature, CG cells start growing and a linear increase with time of the austenite fraction at constant temperature is assumed as described in appendix D. The path followed is schematically shown with the segment A-B in Fig. 8, it corresponds to what can be called a pre-eutectic reaction.31) When the carbon content in the liquid reaches that of the austenite liquidus extrapolation, this “transitory” step ends and solidification then proceeds along b and then c. In the calculations shown in the next section, ΔTtip was set to 10°C.

Same as Fig. 2 but with primary deposition of graphite ending when the temperature equals (TLA-ΔTtip). The solidification path then follows the line segment A-B to reach the metastable extrapolation of the austenite liquidus, and finally continues along b and c.
The values of A1 were evaluated assuming a continuous decrease of the nucleation constant from alloy Ano-inoc to alloy Sno-inoc. Its value was thus first estimated to fit the cell count for alloys Ano-inoc and Sno-inoc, and for a couple of intermediate alloys, and then a smooth variation was set up. Note that the composition and the pouring temperature of each alloy listed previously17) were considered in the calculations. The values used for A1 are plotted in Fig. 9 where are also compared the calculated and experimental CG cell counts. The figure shows a marked decrease of the number of cells from alloy A to alloy E, i.e. the first 80 minutes of holding, and then a smoother decrease. Although the related decrease of A1 was monotonous (see the crosses in Fig. 9), some slight scattering in the calculated cell count is observed which has to do with the variations in alloy composition and pouring temperature.

Experimental and calculated CG cell counts (mm−3) and values of A1 (mm−3·K−1) used for the calculations.
In Fig. 10 is plotted the evolution with time of the calculated and experimental cooling curves for alloy Cno-inoc after the modification of the model discussed in relation with Fig. 6. It is seen that the calculated curve shows now a pre-eutectic plateau which mimics satisfactorily the experimental one. The predicted evolution of the overall solid fraction, VS, the amount of off-eutectic austenite, Vγ,off, and that of CG eutectic, VCG, are also shown. As before, the amount of off-eutectic austenite first increases, then slightly decreases during recalescence and finally increases again. This decrease is related to melting back of some off-eutectic austenite which is necessary for the composition of the remaining liquid to follow the austenite liquidus (stage b) as assumed. It is seen in Fig. 10 that the experimental minimum temperature and amplitude of recalescence are perfectly reproduced by calculations. In order to better reproduce the very end of solidification, the correction factor which allows accounting for impingement of the growing eutectic cells (see appendix C) was changed from the Avrami’s factor ψ used for Fig. 6 to ψ1.5 for this and all other calculations.

Experimental (dotted curve) and calculated (solid curve) cooling curves for alloy Cno-inoc. Also shown are the predicted overall solid fraction, VS, the amount of off-eutectic austenite, Vγ,off, and that of CG eutectic, VCG. (Online version in color.)
Contrary to the case of the first four alloys A-D, all cooling curves for alloys from E to S had a minimum temperature before recalescence below TEW,17) meaning ledeburite could form early during bulk eutectic growth. All the experimental cooling curves were thus scrutinized and it was found that most of them showed a sharp temperature increase of about 3–4°C such as those illustrated with open arrows in Fig. 11. These sharp arrests are much alike that reported by Lueben.32) This feature should be associated to the formation of ledeburite as the growth rate of newly nucleated CG cells could not be high enough for generating such a nearly instantaneous recalescence. What was more surprising is that this thermal arrest could be located anywhere in the first half of the eutectic plateau. This suggested that this arrest relates to the front of a ledeburite cell passing close to the thermocouple junction. In turn, this means that the experimental minimum temperature may show some scattering depending on the actual solidification microstructure developing nearby the thermocouple junction.

Experimental cooling curves of alloys Ono-inoc and Qno-inoc showing a sharp thermal arrest (open arrows) during the eutectic plateau. (Online version in color.)
The above description suggests also that the temperature indicated by the thermocouple could be an average of the temperature at different growth fronts within a few cubic millimeters around the junction. This could explain that the temperature of the eutectic plateau in Fig. 11 is significantly below TEW while growth of ledeburite is expected to proceed at very low undercooling. It was thus concluded that ledeburite did effectively appear in the center of all samples E to S as soon as the temperature felt below TEW, i.e. that there was a negligible nucleation barrier for it.
Figure 12 shows the resulting competition between stable and metastable eutectics in the case of alloy F. After the pre-eutectic plateau, the temperature decreases and the CG cells start growing as seen with the calculated VCG curve. However, the overall solidification rate is yet too low at this stage to avoid the temperature to fall below TEW. Slightly below this temperature, ledeburite is thus predicted to start growing which gives a very small recalescence of about 1°C. For a while, the temperature remains just below TEW while both CG cells and ledeburite are growing. Later, the CG cells have become large enough and the associated heat release makes the temperature overtake TEW. Accordingly, growth of ledeburite then stops and its fraction remains constant during a large part of the eutectic plateau, see the VW curve. At the end of solidification, as the temperature falls again, ledeburite appears again and gives a marked horizontal arrest on the calculated curve which is not seen on the experimental one. This is thought to be due to microsegregation building up as silicon is rejected ahead of the growth front of ledeburite and thus lowering its growth rate, a feature that is not considered in the present modelling approach.

Experimental (dotted curve) and calculated (solid curve) cooling curves for alloy Fno-inoc. Also shown are the predicted overall solid fraction, VS, the amount of off-eutectic austenite, Vγ,off, of CG eutectic, VCG, and that of ledeburite, VW. (Online version in color.)
The fraction of cementite has been measured and it was noticed that the image analysis procedure could not differentiate cementite and ferrite in rod-like ledeburite.17) This means that when the metastable eutectic appeared as clearly separated cementite plates, the values measured were the fractions of cementite, but that when cementite appeared as rod-like ledeburite this was the amount of metastable eutectic that was measured. The comparison which is made of experimental and predicted values in Fig. 13 shows large scattering and discrepancies that are thought to be due to this difficulty in image analysis. However, it is noted that the expected trend of increasing fraction of metastable eutectic with holding time, i.e. with the decrease of the CG cell count, is effectively observed.

Comparison of the measured amount of carbide or ledeburite (see text) and of the predicted amount of ledeburite for the whole series of alloys.
Finally, it is worth checking if the recalecence process which is so typical of CGI is properly reproduced by the calculations. For solidification proceeding in the stable system, Fig. 10 shows a large recalescence. When there is a competition between stable and metastable eutectics as in Fig. 12, most of the recalescence may be associated to growth of the CG cells if the temperature overtakes TEW during the eutectic plateau. However, as the holding time of the melt increases, the number of CG cells decreases and eutectic recalescence fades until the eutectic plateau is nearly flat except for some vertical shift such as illustrated in Fig. 11. Figure 14 presents a comparison of experimental and calculated recalescence values for the whole series of alloys. It is seen that as the number of CG cells starts decreasing, recalescence strongly increases – from alloy Ano-inoc to alloy Dno-inoc – and then decreases when ledeburite precipitates concurrently during bulk eutectic solidification in agreement with previous work.11) The experimental trend is quite well reproduced by the calculations and this comparison lands support to the description of the growth of CG cells that has been adopted in this work.

Comparison of calculated and experimental recalescence values for the whole series of alloys.
The main features of the solidification of not inoculated hypereutectic compacted graphite irons as recorded by thermal analysis could be reproduced with quite a simple approach. Deep undercooling and marked recalescence which are so characteristic of CGI solidification in the stable system appear to be closely related to the proposal that branching of graphite lamellas occurs at the extreme limit of coupled growth. The competition between stable and metastable solidification could be described and leads to a decrease of the recalescence amplitude as compared to a solidification proceeding entirely in the stable system. Owing to the fact that inoculation must be limited when casting CGI, the present work suggests to slightly increase the amount of silicon in these alloys in order to further decrease the metastable eutectic temperature, i.e. to slightly open up the window for stable solidification.
Using the known composition of the alloy, the corresponding austenite liquidus temperature,
| (A-1) |
| (A-2) |
The nature of the alloy, either hypoeutectic or hypereutectic, is determined by the highest of these two temperatures. Further, equating these two temperatures gives the eutectic carbon content,
| (A-3) |
When inserting this value in either of the liquidus expression, one gets the eutectic temperature in the stable Fe–C isopleth section, TEUT. The metastable eutectic temperature, TEW, is expressed as:17)
| (A-4) |
The carbon content of the metastable eutectic is calculated by inserting this temperature in Eq. (A-1) with the other alloying elements set at their nominal value.
Appendix BNucleation sites for graphite are expected to follow a size distribution related to an undercooling distribution for site activation.33) A power law is assumed giving the following relation between the total number of possible sites, NV, at an undercooling
| (B-1) |
It is further assumed that nucleation is instantaneous once the critical undercooling for a particular type of nuclei has been reached, and this means that nucleation stops in case the undercooling
| (B-2) |
The undercooling must be calculated at each time step, which means that the carbon content of the remaining liquid,
| (B-3) |
Furthermore, assuming the liquid has a homogeneous composition, the carbon balance is given by:
| (B-4) |
Combining these two balances gives:
| (B-5) |
Growth of primary graphite spheroids is described considering both diffusion of carbon in the liquid and chemically controlled carbon transfer at the liquid graphite interface. The flux density of carbon ϕ related to this latter chemical reaction is written:
| (B-6) |
| (B-7) |
By combining Eqs. (B-6) and (B-7) and assuming a steady state for carbon distribution in the liquid,
| (B-8) |
Note that the total volume of graphite VG within the representative volume element is given by:
| (B-9) |
K has been set to 0.5 m·s−1 in the present calculations so as to get graphite particles of 5–10 μm in diameter at the beginning of the eutectic reaction.
Appendix CFor the hypereutectic alloys considered in this work, nucleation of graphite particles stops when primary deposition ends, i.e. when the extrapolation of the austenite liquidus is reached (see Fig. 2). Though this is not necessary, the model below is written assuming that all primary nodules have the same size so that an equivalent nodule radius was calculated based on the nodule count, Nm, and the graphite volume, VG,p, at the end of primary deposition:
| (C-1) |
The CG cells start growing from these nodules together with off-eutectic austenite dendrites and metastable eutectic cells if the temperature is below TEW. Following previous works on SGI18) and mottled SGI,33) the overall mass balance is thus given as:
| (C-2) |
| (C-3) |
| (C-4) |
One thus has from Eq. (C-2):
| (C-5) |
Note that in Eqs. (C-2) and (C-5) the volume defined by the CG cells is decreased by VG,p.
On the other hand, the carbon mass balance writes:
| (C-6) |
It is convenient to introduce the following quantity:18)
| (C-7) |
The carbon mass balance may thus be written:
| (C-8) |
To follow the evolution of solidification, the carbon mass balance has to be differentiated with respect to time, noting that
| (C-9) |
For the ledeburite cells, one has:34)
| (C-10) |
The derivative of the carbon mass balance is thus:
| (C-11) |
This equation may be rearranged as:
| (C-12) |
In this equation, the change of VCG and VW are related to the number of cells and their growth rates which have been given in the main text. These volume changes should be weighed to account for impingement, and this was done here using the correction factor ψp, where ψ is the Avrami factor. The exponent p was first set to 1 (Fig. 6) and then to 1.5 for all later calculations. One thus has:
| (C-13) |
| (C-14) |
| (C-15) |
| (C-16) |
Note that the initial value of RCG is RG,eq so that VG,p does not appear in Eq. (C-16) as it is already included in VCG.
The time derivative of ϕ, dϕ/dt, has already been expressed as:18)
| (C-17) |
The change in volume of solid must now be calculated and inserted in the heat balance. According to the above assumptions, this change is written:
| (C-18) |
After differentiation and rearrangement of the last term of the right hand side of Eq. (C-18), one gets:
| (C-19) |
The change in the volume of off-eutectic austenite depends strongly on the change in temperature and this may lead to numerical instabilities in an explicit scheme as used in this work. This suggested introducing in the above equation the expression of dgL/dt from the derivative of the carbon mass balance. Combining Eqs. (C-12) and (C-17) thus gives:
| (C-20) |
Finally, the change in composition of the liquid is assumed to follow the extrapolation of the austenite liquidus, meaning that:
| (C-21) |
Equation (C-21) is then inserted in Eq. (C-20) to give:
| (C-22) |
(C-22) is then substituted in (C-19), then (C-19) in (C-18) to give the change of the volume of solid to be inserted in the heat balance, equation 10 of the main text. After rearranging, one gets:
| (C-23) |
This gives the change in temperature at time t from which is then calculated dgL. The variables are reinitialized at each time step and the calculation pursued until the solid fraction VS/Vt is higher than 0.99.
Appendix DDuring the so-called pre-eutectic step, no metastable eutectic can be formed and the overall mass balance (C-2) may be written:
| (D-1) |
In the same way, the carbon mass balance becomes:
| (D-2) |
From Eq. (D-1) one gets VL which is then inserted in Eq. (D-2), giving:
| (D-3) |
In the simple approach adopted here, the temperature was assumed constant during the pre-eutectic stage. The procedure is to calculate the volume change of the CG cells and to increment the volume of austenite by a predefined amount dVγ until the carbon content in the liquid becomes equal to the value at the austenite liquidus at the corresponding temperature. An estimate of the volume of austenite allowing for the change of the liquid content in carbon along A-B in Fig. 8 is obtained by applying the lever rule for the carbon content at the end of primary precipitation of graphite, point A in Fig. 8. With a time step set to 0.01 s, dVγ was then set to 10−3 times this amount.