2022 年 62 巻 6 号 p. 1258-1267
2022 年 62 巻 6 号 p. 1258-1267
Effects of Zr addition on the microstructure formation of two intermetallic phases of Fe2Nb Laves (TCP) and Ni3Nb (GCP) in a carbon-free Fe-20Cr-35Ni-2.5Nb (at.%) novel austenitic heat-resistant steel at elevated temperatures were examined from the viewpoints of thermodynamics and kinetics. From a thermodynamic perspective, the maximum solubility of Zr in the matrix phase is about 0.1 at.% under homogenization treated states at around 1473 K. The dissolved Zr in the matrix eventually reduces the solubility limit of Nb, thereby playing an important role in promoting the formation of grain-boundary Fe2Nb phase as well as that of thermodynamically stable Ni3Nb-δ phase within grain interiors after long-term aging at 1073 K. The Zr dissolved in the matrix after the homogenization treatment is found to enrich in δ phase formed during the aging and raises the temperature limit of formation of δ phase by about 50 K. From a kinetic perspective, because of the increase in Nb supersaturation, the dissolved Zr not only enhances the precipitation of Laves phase at grain boundaries but also promotes the homogeneous nucleation of metastable Ni3Nb-γ″ phase within grain interiors from the beginning of aging and retards the phase transformation from γ″ to δ. These microstructure changes by Zr can be interpreted in terms of the phase equilibria and relative phase stability among different phases. The present results reveal that even a small amount of Zr in solution, not segregation to grain boundaries, has tremendous effects on the precipitation behavior and morphology of intermetallic phases.
To improve the energy efficiency of a coal-fired power plant and reduce CO2 emission it is necessary to raise the steam temperature in the boilers.1,2,3,4) In an advanced ultra-supercritical (A-USC) thermal power plant for the next generation operated above 973 K,5) a 105 h creep-rupture strength of 100 MPa or higher3,4) is required for the boiler tubes/pipes and turbine rotor components. Many Ni-based superalloys strengthened by Ni3Al-γ′ phase3,4,6,7,8) have been developed as candidate for the A-USC power plant components. On the other hand, the steels strengthened by transition metal carbides do not fulfill the requirement.3,4,9,10) However, Takeyama et al. have recently proposed a new concept to develop novel carbon-free austenitic heat-resistant steels strengthened by two intermetallic phases of topologically close-packed (TCP) Fe2Nb Laves and geometrically close-packed (GCP) Ni3Nb,11,12,13,14,15,16,17,18,19,20,21,22,23) based on thermodynamics and precipitation kinetics aspects. They found that the allowable temperature of 105 h creep-rupture strength under 100 MPa in the novel steels can exceed 973 K and the temperature is almost comparable to that of a candidate nickel-base alloy Nimonic 263.24,25) The superior creep-rupture strength of the novel steels is mainly due to the precipitation of TCP Fe2Nb Laves phase at grain boundaries (GBs) named as the “Grain-boundary Precipitation Strengthening (GBPS)” mechanism,11,12,15,16,17,18,19,20,21,22,23,26) together with the precipitation of metastable Ni3Nb-γ″ and subsequent stable Ni3Nb-δ phase within grain interiors.18)
The addition of Zr is reported to enhance the creep-rupture strength of these novel steels.13) In general, Zr is widely accepted as a grain-boundary strengthener in heat resistant alloys27,28,29,30) due to segregation to grain boundaries because of its large atomic size with respect to the host atom.31) However, in our previous study, the small amount of Zr additions to one of the novel steels based on Fe-20Cr-30Ni-2Nb (at.%) was found to enhance the grain-boundary precipitation of the Fe2Nb Laves phase.18) Thus, the strengthening of the steel by the Zr addition could be associated with the increase in the precipitation of GB Laves phase based on the GBPS mechanism. In addition, we also revealed that Zr was enriched in the metastable Ni3Nb-γ″ phase precipitated within the grain interiors and retarded the transformation to the stable Ni3Nb-δ phase.18) These results suggest that the solid solution of Zr atoms in the matrix somehow affects the precipitation behavior of the intermetallic phases, although the grain-boundary segregation of Zr cannot be ruled out.
In this study, thus, several carbon-free novel austenitic steels containing Zr up to 0.5 at.% were prepared, and the effects of Zr addition on the precipitation and stability of TCP Fe2Nb Laves and GCP Ni3Nb phases were examined from thermodynamics and kinetics viewpoints, for the sake of further development of the novel steels.
Three steels with a base composition of Fe-20Cr-35Ni-2.5Nb (all compositions are at.% unless otherwise stated) containing a different amount of Zr up to 0.5 at.% were used in this study. The nominal compositions and the heat treatments employed to these steels are summarized in Table 1. These steels are hereafter referred to as base (no Zr addition) and Zr content. The 30 g button ingots of each composition were prepared by arc melting under argon atmosphere using the metals of 99.9% purity. During melting process each ingot was turned over several times to eliminate un-melted metals and to homogenize the mixture. The weight loss of each ingot after the melting is less than 0.1 g. The ingots were first homogenized to eliminate the microstructure inhomogeneity occurring during solidification and then cold-rolled to about 30% in height reduction. The cold-rolled ingots were then homogenized again to adjust the grain size to about 150 to 200 μm, followed by water quench. Subsequently, the homogenized specimens were aged at given temperatures up to 3600 h.
| Steel | Nominal composition/at.% | Homogenization treatment | Aging conditions | ||||
|---|---|---|---|---|---|---|---|
| Cr | Ni | Nb | Zr | Fe | 973 K–1173 K 12 s–3600 h | ||
| Base | 20 | 35 | 2.5 | – | bal. | 1523 K/1 h | |
| 0.1Zr | 20 | 35 | 2.5 | 0.1 | bal. | 1463 K/2 h | |
| 0.3Zr | 20 | 35 | 2.5 | 0.3 | bal. | 1443 K/4 h | |
| 0.5Zr | 20 | 35 | 2.5 | 0.5 | bal. | ||
* Aging for up to 15 minutes was done by salt bath.
For microstructure observations, the heat-treated specimens were mechanically polished, followed by electro-polishing in a solution of phosphoric acid containing supersaturated chromic anhydride. The microstructures were examined using a field emission scanning electron microscope (FE-SEM) with an acceleration voltage of 10 to 15 kV with a working distance of 10 mm. The compositions of the phases present in the heat-treated specimens were analyzed using a field-emission electron probe microanalyzer (FE-EPMA) equipped with wave-length dispersive spectrometer (WDS) under 15 kV and 20 nA. The compositions were determined by point analysis at 10 different positions for each phase and the areas analyzed were larger than 1 μm to eliminate the broadening effect. The data with the total mass of 100 ± 2 wt.% were averaged with an accuracy of ± 0.01 wt.%. The elemental mappings were also performed for thin or small phases to qualitatively analyze the compositions with a step size of 0.06 μm and dwell time of 10 ms.
The grain-boundary area fraction of precipitates (ρ), which is defined as the grain-boundary area decorated with precipitates with respect to the total grain boundary area, was quantified using the following Eq. (1):
| (1) |
Figure 1 shows the secondary electron images (SEIs) for the change in microstructures with the increase in Zr content of the steels aged at 1073 K for the longest aging time of 3600 h, closest to the equilibrium state. In the base steel (Fig. 1(a)), as previously reported,26) the grain boundaries are decorated mostly by the spheroidal Fe2Nb (C14) Laves phase, together with a small amount of Ni3Nb-δ (D0a) phase, and the area fraction ρ is about 60%. Within the grain interiors, two kinds of precipitates with a short ellipsoidal shape and a long needle-like shape are uniformly distributed, and they are respectively identified as the Laves and δ phases. In the Zr added steels (Figs. 1(b) to 1(d)), the grain boundaries are obviously covered with globular shaped precipitates with a size of 1 to 2 μm, and the ρ values become higher than that of the base steel, regardless of the Zr content. However, some large particles with about 5 μm in size are often observed at the grain boundaries with increasing Zr content. Within grain interiors, the addition of 0.1 at.% Zr increases the precipitation density of the thicker and elongated precipitates forming the Widmanstatten morphology (Fig. 1(b)). The precipitation morphologies in the Zr added steels are almost the same, regardless of the Zr content. The measured ρ and Hv values are plotted as a function of the Zr content, as shown in Fig. 2. The ρ and Hv values increase from 60% to 74% and from 2.0 GPa to 2.3 GPa, respectively, by adding 0.1% Zr, and the both values remain almost unchanged with a further increase in Zr content.

Secondary electron images (SEIs) showing microstructures of (a) base, (b) 0.1Zr, (c) 0.3Zr, and (d) 0.5Zr aged at 1073 K for 3600 h.
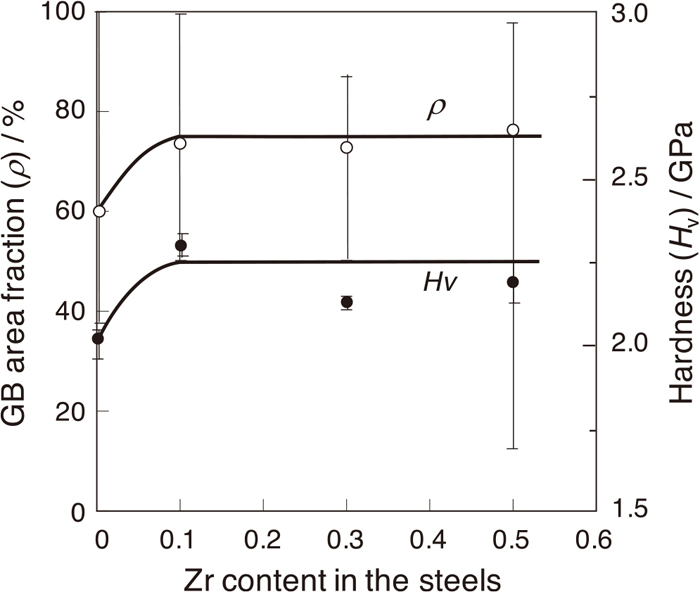
Change in the area fraction covered by the Fe2Nb ρ and the hardness Hv within grain interiors with Zr content in the steels aged at 1073 K for 3600 h.
Figure 3 shows the elemental mapping corresponding to the microstructure of the 0.1Zr shown in Fig. 1(b). At grain boundaries, most of the precipitates are depleted in Fe, Cr and Ni, and enriched in Nb with respect to the γ (fcc) matrix phase, indicating that they are the Laves phase, based on our phase diagram study.14) However, the precipitate formed at the triple junction is enriched in Ni and Nb and further depleted in Fe and Cr, indicating the Ni3Nb-δ phase. It is important to note that Zr is enriched in the region where the δ phase is formed. Within grain interiors, as indicated by arrows the needle-shaped precipitates having a straight habit plane with three directions, forming the Widmanstatten morphology in each grain, are identified as the δ phase where Ni and Nb are enriched and Fe and Cr are depleted. Note that Zr is enriched in the δ phase, in good agreement with the previous report.18) The elongated thicker precipitates formed on and along the δ phase are identified as the Laves phase where Fe, Cr and Ni are depleted but Nb is enriched. There is no indication of Zr enrichment in the Laves phase.

Elemental mappings of the 0.1Zr aged at 1073 K for 3600 h.
From a thermodynamic viewpoint, the present results clearly demonstrate that the addition of only 0.1 at.% Zr to the base steel is effective in enhancing the formation of Fe2Nb Laves phase at the grain boundaries and Ni3Nb-δ phase within grain interiors at 1073 K. The Zr addition seems to enhance the formation of grain-boundary (GB) δ phase as well, as shown in Fig. 3. However, we confirmed that the amount of GB δ phase is still small, so that the increase in the ρ shown in Fig. 2 is mainly due to the formation of the Laves phase. In addition, an important finding is that Zr is prone to enrich in the Ni3Nb-δ phase.
In order to understand the reason why such a small amount of Zr is effective in increasing the formation of Fe2Nb phase at the grain boundaries as well as Ni3Nb-δ phase within the grain interiors, the compositions of γ matrix phase in the specimens after the homogenization treatment (HT) and those in the same specimens aged at 1073 K shown in Fig. 1, together with some precipitated phases, were analyzed, and the results are summarized in Table 2. From these datasets, the concentrations of Nb and Zr dissolved in the matrix are plotted as a function of the Zr content in the steels in Fig. 4. In the HT state, the solubility of Nb in the matrix phase is nearly the same level of 2.3 at.% to 2.4 at.%, regardless of the Zr contents in the steels studied. At 1073 K, however, the solubility of Nb in the matrix phase in the base steel is 0.8 at.%, and it decreases down to about 0.6 at.%, regardless of the Zr content (Fig. 4(a)). Thus, the Nb supersaturation, ΔXNb, at 1073 K is significantly increased by the Zr additions. The solubility of Zr in the matrix phase in the HT state is about a level of 0.07 at.% to 0.08 at.%, regardless of the Zr content in the steels studied, and it is reduced down to about 0.02 at.% at 1073 K (Fig. 4(b)). Note that the large round-shaped particles observed in both 0.3Zr and 0.5Zr steels (Figs. 1(c), 1(d)) are found to contain about 13 at.% Zr and 8 at.% Nb (Table 2), indicating that more than 0.1 at.% addition of Zr to the base steel induces the formation of a highly Zr enriched phase, presumably Ni5Zr (C15b) phase, as a primary solidification phase. Thus, the solubility limit of Zr in the matrix at the HT temperature regions is about 0.08 at.%, and it is about 0.02 at.% to 0.03 at.% at 1073 K.
| Steel | Heat treatment | Phase present | Compositions/at.% | ||||
|---|---|---|---|---|---|---|---|
| Cr | Ni | Nb | Zr | Fe | |||
| Base | HT | γ | 20.3 | 34.5 | 2.30 | – | bal. |
| 1073 K/3600 h | γ | 21.2 | 34.0 | 0.80 | – | bal. | |
| ε | 13.5 | 24.5 | 25.7 | – | bal. | ||
| δ | 3.40 | 67.1 | 21.0 | – | bal. | ||
| 0.1Zr | HT | γ | 21.2 | 34.5 | 2.42 | 0.07 | bal. |
| 1073 K/3600 h | γ | 22.2 | 33.7 | 0.63 | 0.02 | bal. | |
| ε | 14.2 | 24.1 | 25.6 | 0.01 | bal. | ||
| δ | * | * | * | * | * | ||
| 0.3Zr | HT | γ | 21.4 | 34.1 | 2.38 | 0.08 | bal. |
| 1073 K/3600 h | γ | 22.4 | 33.5 | 0.63 | 0.02 | bal. | |
| ε | * | * | * | * | * | ||
| δ | * | * | * | * | * | ||
| Primary | 2.97 | 64.5 | 8.21 | 12.5 | bal. | ||
| 0.5Zr | HT | γ | 21.7 | 33.8 | 2.26 | 0.08 | bal. |
| 1073 K/3600 h | γ | 22.6 | 33.4 | 0.61 | 0.03 | bal. | |
| ε | 14.0 | 24.7 | 25.0 | 0.05 | bal. | ||
| δ | * | * | * | * | * | ||
| Primary | 2.76 | 65.2 | 7.77 | 13.0 | bal. | ||

Solubility of (a) Nb and (b) Zr dissolved in the γ matrix at the homogenization treated state (HT) and at 1073 K as a function of the Zr content in the steels.
In general, Zr is believed to segregate to grain boundaries,29) because of the larger atomic radius (1.602 Å), 15% larger than those of the major matrix constituent elements of Fe (1.274 Å), Ni (1.246 Å) and Cr (1.282 Å) atoms under the CN (coordination number) of 12.31,32) Therefore, a small amount of Zr of about 0.1 wt.% is often added to heat resistant materials as a grain boundary strengthener.29) However, the present result clearly demonstrates that most of the Zr atoms are existing in solution in the γ matrix, rather than segregating to the grain boundaries. Thus, it is evident that the increase in the ΔXNb by the Zr addition gives rise to the increase in the ρ. The increase in the ΔXNb also plays an essential role in increasing the precipitation density of Ni3Nb-δ phase within the grain interiors since Nb is a main constituent element of the δ phase. However, the Zr supersaturation, ΔXZr, also contributes in enhancing the formation of the δ phase at 1073 K, as shown in Fig. 4(b), since Zr is enriched in the δ phase.
The decrease in the solubility of Nb in the γ matrix phase is related to the Zr in solution. We tentatively calculated the lattice parameters a of the matrix phase in the base and 0.1Zr steels using the analyzed compositions of the elements in Table 2, based on the hard sphere model (
The increase in ΔXNb by the Zr addition would raise the temperature limit of the thermodynamically stable δ phase. Figure 5 shows an elemental mapping of the microstructures of the base and 0.1Zr steels aged at 1123 K for 1460 h. This aging time is far beyond the aging time of 3600 h at 1073 K, according to Larson-Millar conversion. In both steels, the grain-boundary precipitates are the Laves phase where Ni is depleted and Nb is enriched, and the ρ is higher in the Zr added steel. Within the grain interiors, in the base steels all of the precipitates with an elongated shape of 2 μm in length is the Laves phase where Ni is depleted and Nb is enriched (Fig. 5(a)). In Zr added steel, most of the precipitates with the same shape are also the Laves phase, and the precipitation density becomes higher than that in the base steel. Besides the laves phase, some needle-like precipitates indicated by yellow arrows are identified as Ni3Nb-δ where Ni, Nb and Zr enriched (Fig. 5(b)). The result of the increase in upper temperature limit of the formation of thermodynamically stable Ni3Nb-δ phase by Zr addition indicates that Zr addition stabilized the δ phase. It is also noteworthy that long needle-like precipitates pointed by red arrows in Zr added steels (Fig. 5(b)) are the Laves phase, not the δ phase, and the precipitation morphology of the Laves phase by Zr additions obviously changes to Widmanstatten-type at 1123 K.

Elemental mappings of (a) base and (b) 0.1Zr aged at 1123 K for 1460 h.
In the previous section, regardless of the Zr content of the steels, the 0.1 at.% Zr dissolved in the matrix plays an important role in enhancing the formation of Fe2Nb at the grain boundaries and that of Ni3Nb-δ phase within the grain interiors, from the thermodynamics viewpoint. This section focuses on the 0.1Zr steel and how the Zr in solution affects the precipitation behavior of both intermetallic phases, from the kinetics viewpoint.
Figure 6 shows a typical example of the change in the precipitation morphology with Zr addition at different aging time at 1073 K in the base and 0.1Zr steels. In the base steel after 1 h aging, the elongated Fe2Nb Laves phase with an average length of 1 μm is non-uniformly precipitated at the grain boundaries, and no precipitates are observed within the grain interiors (Fig. 6(a)). After 24 h aging, the GB Laves phase becomes larger and the ρ is still less than 50%. Within the grain interiors, three different shapes of inhomogeneously distributed precipitates are observed; very fine dotted particles (< 500 nm) identified as metastable Ni3Nb-γ″ (D022) phase,26) needle-shaped precipitates (1 to 2 μm) identified as stable Ni3Nb-δ (D0a) phase transformed from the γ″ phase, and ellipsoidal particles (1 μm) identified as Fe2Nb (Fig. 6(b)). In contrast, in the 0.1Zr steel, the finer GB Laves phase particles are homogeneously distributed, and the ρ apparently increases even after 1 h. Within the grain interiors, the very fine γ″ precipitates are already formed and homogeneously distributed even after 1 h aging (Fig. 6(c)). After further aging of 24 h, the uniform distribution of the GB Laves phase particles are kept and the ρ is much higher than that in the base steel. Within the grain interiors, the γ″ phase grows to some extent and the transformed product of needle-shaped δ phase is developed. In addition, thick elongated precipitates identified as Fe2Nb are also formed (Fig. 6(d)). Then, the quantitative analyses of the ρ and Hv in the base and 0.1Zr steels aged for various aging times were preformed, and the values are plotted as a function of aging time in Fig. 7. In both steels, the ρ values progressively increase with increasing the aging time and reach the maximum values shown in Fig. 2 after 1200 h aging. The value of ρ in the Zr added steel becomes higher by approximately 14% from the beginning of the precipitation to the entire aging to 3600 h (Fig. 7(a)). In the case of Hv within the grain interiors (Fig. 7(b)), both steels show nearly the same values of 1.5 GPa in the HT state. In the base steel, the Hv starts increasing after aging for a few hours and reaches a peak of 2.2 GPa at about 100 h, followed by a gradual decrease to 2.0 GPa to 3600 h. In the 0.1Zr steel, the Hv starts increasing after aging for a few minutes by an order of magnitude faster than that in the base steel and rapidly increases to a peak of 2.8 GPa at around 10 h, followed by a slight decrease to 2.3 GPa with aging to 3600 hs. It is worth mentioning that the Hv value doubles after the few hours of aging by adding only 0.1 at.% Zr to the base steel.

SEIs showing the difference in microstructure of (a, b) base and (c, d) 0.1Zr aged for 1 h (a, c) and 24 h (b, d) at 1073 K.

Change in (a) ρ and (b) Hv within the grain interiors with aging time in the base and Zr added steels at 1073 K.
The same microstructure analyses were done at 1123 K, and the change in ρ and Hv with aging time is shown in Fig. 8. Both steels show similar trends of ρ with aging time, although the values of the Zr added steel become higher by approximately 10% than that in the base steel during the entire aging. The ρ values after aging for 1200 h at 1123 K is higher than those at 1073 K for 3600 h (Fig. 8(a)). The Hv values in the both steels also show the similar trend (Fig. 8(b)), although the increased value of the peak hardness by the Zr addition becomes smaller than that obtained at 1073 K (Fig. 7(b)). The corresponding microstructures before and at the peak hardness for both steels are shown in Fig. 9. Before the peak of 1 h aging (Figs. 9(a), 9(c)), the base steel shows only the GB Laves phase sparsely precipitated and almost no precipitates within the grain interiors, whereas the Zr added steel shows the GB Laves phase covered most of the grain boundaries and very fine γ″ phase particles precipitated within the grain interiors either homogeneously or with aligned morphology depending on the grains. At the peak of 24 h aging (Figs. 9(b), 9(d)), although the morphology of the GB Laves phase is nearly the same, the precipitation morphology within the grain interiors is distinctly different between the two steels. The base steel shows a sparse distribution of ellipsoidal particles identified as the Laves phase, whereas the Zr added steel exhibits the Widmanstatten morphology consisting of thick and elongated precipitates with 5 μm in length. The elongated precipitates were identified as the Laves phase where Fe is enriched, although some amount of thin needle-shaped δ phase with about 1 μm in length exists. Note that the precipitation density of the Laves phase in the Zr added steel is higher than that in the base steel, and this is responsible for the increase in the Hv values by the Zr addition (Fig. 8(b)).

Change in (a) ρ and (b) Hv within grain interiors with aging time in the base and Zr added steels at 1123 K.

Microstructure change with aging time at 1123 K for (a, c) 1 h and (b, d) 24 h, nearly the peak hardness in Fig. 8(b), of the base (a, b) and the 0.1Zr (c, d).
Based on these results, effects of Zr addition on the precipitation kinetics of TCP Laves phase and metastable and stable GCP Ni3Nb phases are depicted as time-temperature precipitation (TTP) diagram together with the TTP in the base steel26,33) in Fig. 10, where the εGB and εGI are the Laves phase precipitated at the grain boundaries and within the grain interiors, respectively. There are several points to be noted on Zr effects on the precipitation kinetics. Firstly, the Zr addition accelerates the formation of GB Laves phase (Ps (εGB)) by nearly an order of magnitude. Secondly, the Zr addition raises the nose temperature of the C curve of the formation of γ″ phase within the grain interiors (Ps (γ″)) by about 50 K and also accelerates the formation of γ″ phase by an order of magnitude. Thirdly, the Zr addition also raises the nose temperatures of the C curves of the start (TS) and finish (Tf) of the γ″ to δ phase transformation by about 50 K and enhances the phase transformation kinetics above the nose temperature but slows it down below the temperature. Lastly, the Zr addition retards the formation of the Laves phase within the grain interiors (Ps (εGI)), because of the consumption of the ΔXNb by the formation of γ″ and δ phases.

In section 3.1, the addition of 0.1 at.% Zr to the base steel of Fe-20Cr-35Ni-2.5Nb revealed to bring on the following three distinct changes from the thermodynamic viewpoint: decreasing the Nb solubility in the γ matrix (Fig. 4), increasing the ρ of the GB Fe2Nb Laves phase (Fig. 2) and stabilizing the Ni3Nb-δ phase by nearly 50 K up to about 1073 K (Fig. 5). These changes can be interpreted by the relative phase stability change among γ, Fe2Nb-ε (TCP) and Ni3Nb-δ (GCP) phases.14) Figure 11 shows a schematic illustration of the Gibbs energy/composition diagram of the three phases at a given temperature. In the case of the steel without Zr, the terminal compositions of the three phases in equilibrium each other, so called “the three-phase tie triangle” can be obtained from the common tangent plane among the free energy curvilinear surfaces of the three phases, depicted as a solid line at the bottom of Fig. 11. Since Zr is identified to enrich in the δ phase, the free energy of the δ phase should be decreased. The curvature of the free energy curvilinear surface of the γ phase is larger than those of the intermetallic phases, so that the decrease in the energy of the δ phase makes the contact point (solid circle) of γ phase on the common tangent plane further away toward Ni-(Fe+Cr) line, thereby changing the three-phase tie triangle as shown by the dotted line in Fig. 11, resulting in the decrease in solubility of Nb in the matrix phase. This is responsible for the increase in the ρ of GB Laves phase. The change in the three-phase tie triangle also makes it possible to change the phase equilibria from the γ+ε two-phase region to the γ+ε+δ three-phase region in a certain alloy composition range as shown in the solid red circle.

Schematic illustration showing the change in the phase equilibria among γ, Fe2Nb-ε (TCP) and Ni3Nb-δ (GCP) phases by the addition of Zr, based on a thermodynamic viewpoint. The solid line is for the base steel system and the broken line for the Zr added system. (Online version in color.)
In section 3.2, from the kinetic viewpoint, the addition of 0.1 at.% Zr to the base steel was shown to enhance the formation of metastable Ni3Nb-γ″ (GCP) phase within the grain interiors in short-term aging (Figs. 6, 7, 8, 9), prior to the formation of stable Ni3Nb-δ phase after long-term aging. Formation of this metastable γ″ phase is commonly observed in Ni-base alloys containing Nb, such as IN718, although the mechanism of formation has not been uncovered yet. The enhancement of the formation of γ″ phase in this study is associated with the decrease in Nb solubility in the matrix, that is, the increase in Nb supersaturation, ΔXNb. Figure 12 shows a schematic illustration showing the γ+ε+δ three-phase tie triangles of the steels without and with Zr at a given temperature, depicted based on the thermodynamic consideration of Zr effects shown in Fig. 11, where the terminal composition of the metastable γ″ phase is considered the same as that of the δ phase. This assumption is reasonable since the transformation (γ″→δ) is the second order transformation and the Zr is already enriched in the γ″ phase.18) Now let us consider the decomposition process of the supersaturated γ phase (γss) at the point O during aging at the given temperature, where the point O in the three-phase regions is a fixed alloy composition in both steels after the homogenization treatment. In both steels, the Laves phase forms first mainly at the grain boundaries, followed by the γ″ formation within the grain interiors (Fig. 10). Suppose that the ΔXNb is maintained in the regions away from the grain boundaries, the formation of metastable γ″ phase would occur along the following decomposition process:

Schematic illustration showing the decomposition processes of the supersaturated γ phase for the formation of Fe2Nb Laves (TCP) and Ni3Nb (GCP) phases, based on thermodynamic and kinetic viewpoints. (Online version in color.)
As aging proceeds, the following two reactions take place:
It is interesting to discuss the change in the precipitation morphology of the Fe2Nb Laves phase within the grain interiors from a random-distribution type consisting of an ellipsoidal shape to the Widmanstatten-type consisting of a needle-like shape (Figs. 5 and 9(b), 9(d)). This is associated with the transformation process of GCP and TCP phases as mentioned above. Figure 13 schematically illustrates how the precipitation morphology of the Fe2Nb Laves phase becomes Widmanstatten-type in terms of the precipitation sequence of the precipitates within a grain interior with aging, particularly the case where the <111>γ orientation of the grain is normal to the observation surface (paper plane). In the beginning of aging, metastable γ″ phase homogeneously precipitates (Fig. 13(a)), and because of the difference in the precipitation habit plane against γ matrix phase between the γ″ phase ((001)γ″//{001}γ, <010]γ″// <100>γ) and δ phase ((010)δ //{111}γ, [100]δ // <011>γ), the γ″ phase transforms to the δ phase with aligning along the four {111} planes of the γ phase, as shown in Figs. 6(c) and 9(c). Once the transformation is completed, the precipitation morphology of the δ phase becomes the Widmanstatten-type (Figs. 13(a), 13(b)). This is because the δ phase precipitates with a platelet shape having the above crystallographic orientation relationship, so that in most cases the δ phase is visible along three different line directions intersecting the four {111}γ planes on the observation surface for each grain, except the grain with <100>γ orientation perpendicular to the observation surface where only two orthogonal intersection line directions exist. Since the δ phase can dissolve less Fe and Cr atoms (Table 2), the formation of δ phase results in the enrichment of these atoms locally around the δ phase, enhancing the nucleation of Laves phase at the δ/γ interfaces (Fig. 13(b)). In case that the precipitation density of the γ″ phase is small, the length of the δ phase is limited so that the further transformation from δ to ε results in a random distribution morphology of the Laves phase. On the other hand, if the precipitation density is high like the case in the present steel with Zr, the transformed morphology of the δ phase becomes Widmanstatten-type consisting of long platelet shape, so that the further transformation from the δ phase to ε phase gives rise to the unique Widmanstatten-type morphology of the Laves phase. The morphology change in the Laves phase thus suggests that the γ″→δ phase transformation preferentially takes place prior to the precipitation of the Laves phase within the grain interiors in the composition and temperature ranges where both δ and ε phases form within the grain interiors during aging.

Schematic illustration showing how the precipitation morphology of the Fe2Nb Laves phase becomes Widmansttaten-type, in terms of the transformation sequence of the precipitates within the grain interiors during aging: (a) Ni3Nb-γ″, (b) Ni3Nb-δ, (c) Fe2Nb-ε phase. The lines represent the intersection of four {111}γ planes on the observation surface in a grain with <111>γ orientation perpendicular to the observation surface.
The effects of Zr addition on the precipitation behavior and morphology of the Fe2Nb Laves (TCP) phase and Ni3Nb (GCP) phases in a carbon-free Fe-20Cr-35Ni-2.5Nb novel austenitic heat-resistant steel have been investigated from viewpoints of thermodynamics and kinetics, and the following conclusions can be drawn:
(1) The maximum solubility of Zr in the fcc matrix in the novel steel is about 0.1 at.% and 0.02 at.% at around 1473 K and 1073 K, respectively. The further addition of Zr induces a Zr enriched primary solidification phase.
(2) The Zr dissolved in the matrix decreases the Nb concentration in the fcc matrix phase in equilibrium with the Laves and δ phases, thereby increasing the Nb supersaturation for the formation of the intermetallic phases.
(3) The Zr dissolved in the matrix at 1473 K is mostly enriched in the Ni3Nb-δ phase formed by aging at 1073 K, and the temperature limit of thermodynamically stable δ phase increases to 1123 K, nearly 50 K higher that in the steel without Zr.
(4) At the grain boundaries, the Zr dissolved in the matrix is effective in promoting the precipitation of grain-boundary Fe2Nb Laves phase from the biginning of the aging, and the grain-boundary area fraction covered by the Laves phase in the steel with 0.1 at.% Zr becomes higher by at least 10% than that in the steel without Zr at 1073 K and 1123 K.
(5) Within the grain interiors, the Zr dissolved in the matrix enhances the homogeneous nucleation of metastable Ni3Nb-γ″ phase in the early stage of aging at 1073 K and 1123 K, and the formation of transformed Ni3Nb-δ phase from the γ″ phase is eventually enhanced as the aging proceeds. The further aging promotes the formation of ε phase at the γ/δ interfaces, and this is more obvious at 1123 K.
(6) The formation sequence from γ″→δ→ε phase within the grain interiors drastically changes the precipitation morphology of the Laves phase from a random distribution type to Widmanstatten-type by Zr addition.
(7) From these results, it is Zr in solution, not segregation to the grain boundaries, that causes the change in precipitation microstructures of TCP and GCP phases at grain boundaries and within the grain interiors, even though the amount of dissolved Zr in the matrix is very small. These findings on the Zr effects would make great contributions to construct the microstructure design principle to further improve the high-temperature strength of the novel steels.
This study was supported by the Grant-in-Aid (JY240118) on the Advanced Low Carbon Technology Research and Development Program (ALCA), Japan Science and Technology Agency (JST).