2022 年 62 巻 8 号 p. 1731-1740
2022 年 62 巻 8 号 p. 1731-1740
The effect of blasting on hydrogen analysis was investigated with the aim of establishing a hydrogen analysis method for precisely measuring hydrogen that entered steel in a corrosive environment. The hydrogen existing states of the specimens blasted under various conditions were analyzed using thermal desorption analysis and the hydrogen visualization method by secondary ion mass spectrometry. The phenomenon of hydrogen entry into steel by blasting was demonstrated for the first time. It should be noted that the effect is remarkable in the case of a specimen with a large specific surface area, and the blasting becomes an inhibitory agent in the measurement of the hydrogen content in steel. The hydrogen source for increasing the hydrogen content due to blasting is mainly the water contained in the abrasive. The mechanism of increasing the hydrogen content in steel by blasting is that the fresh surface of the steel exposed by blasting reacts with the water in the abrasive, which results in the hydrogen generation and entry into steel. Additionally, the water in the abrasive remaining on the steel surface reacts with steel during the thermal desorption analysis to release hydrogen. To suppress the increase of hydrogen content by blasting, it is effective to use abrasive with low water content and to remove rust by repeating a short blasting time in order to suppress the temperature rise of the specimen.
To reduce the environmental burden, reducing CO2 emissions from vehicles including automobiles is an important issue. The use of high strength material is one of the effective means for lightening these vehicles. Steel has many advantages such as high strength, low cost, and high workability compared to other materials; however, the susceptibility of steel to hydrogen embrittlement increases with increasing strength of steel. Thus, the suppression of hydrogen embrittlement is the biggest issue for practical use. Hydrogen embrittlement is caused under specific conditions that combine material, environmental, and stress factors.1,2) To improve hydrogen embrittlement resistance, there have been many attempts from various viewpoints such as alloy design, including reduction of P and S which are causes of grain boundary fracture; increase in tempering temperature to control grain boundary carbides; and grain refinement by adding elements such as Ti and V.3,4,5,6)
For considering the practical use of high strength steel, the hydrogen embrittlement evaluation method, which takes into consideration the actual environment and load stress, is indispensable. Several hydrogen embrittlement evaluation methods have been proposed, such as the atmospheric corrosion test,7,8) the JIS draft method for bolt steels,1) methods based on the hydrogen content,9,10,11,12) the combined cycle test,13) and methods based on intrinsic factors.14) The atmospheric corrosion test is an evaluation method close to the actual environment but the evaluation takes a long time. The JIS draft method was proposed as an accelerated test in the laboratory, but it has been reported that the results do not match those of the atmospheric corrosion tests.1,10) Recently, various methods based on hydrogen content measurement have been proposed. For example, Suzuki et al. confirmed that there was critical hydrogen content where hydrogen embrittlement occurred using thermal desorption analysis, and Yamasaki and Takahashi proposed a method of hydrogen embrittlement susceptibility by comparing the critical hydrogen content and the hydrogen content absorbed from the environment. However, a standard method of hydrogen embrittlement has not yet been established, and it is one of the challenges of hydrogen embrittlement research. In the atmospheric corrosion test and the methods based on hydrogen content, it is necessary to accurately evaluate the hydrogen content absorbed from the corrosive environment. In addition, it is ideal to clarify hydrogen content absorbed from the actual environment for considering the practical use.
It has been reported that the hydrogen absorbed from the actual environment due to the atmospheric corrosion is relatively small amount, on the order of 10−2−10−1 ppm.15,16,17,18) In high-strength steel, hydrogen embrittlement is caused even with a small hydrogen content of 0.1 ppm or less, so a precise measurement of hydrogen content is required. Recent advances in analytical technology have made it possible to perform precise hydrogen analysis. For example, by thermal desorption analysis, the hydrogen content can be evaluated on the order of 0.01 ppm. However, the effect of the sample pretreatment prior to hydrogen analysis has hardly been reported, and there is much room for study. In order to accurately evaluate the hydrogen content absorbed from the environment, it is important to understand and remove the inhibitory factors in measuring the hydrogen content. The effect of rust can be mentioned as a typical inhibitory factor in hydrogen content measurement. It has been reported that if the hydrogen content is measured by thermal desorption analysis using steel with rust, hydrogen derived from rust is generated, and the hydrogen content of steel may be overestimated.19) Mechanical polishing is used to remove rust, and blasting is often used for specimens with complex shapes.8,15,18) However, there are no reports investigating the effect of blasting on hydrogen content measurement.
In order to accurately measure the amount of hydrogen absorbed in steel due to atmospheric corrosion, this study examined the effect of blasting, which removes iron rust caused by corrosion, on the measurement of hydrogen content. Then, for the first time, the phenomenon of hydrogen entry into steel by blasting was clarified. Using thermal desorption analysis and the hydrogen visualization method by secondary ion mass spectrometry (SIMS), the hydrogen existing state in steel blasted under various conditions was analyzed, and the mechanism and suppression method of hydrogen entry by blasting was investigated.
SCM 435 steel plate in Japanese Industrial Standards (JIS) was used in this study. Table 1 shows the chemical composition of steels. Steel A is commercially available, and Steel B was prepared in a vacuum induction furnace. The steel was quenched at 900°C for 15 minutes and tempered at 400°C for 30 minutes to obtain a tempered martensitic structure with a tensile strength (TS) of 1500 MPa class.
| Steel | C | Si | Mn | P | S | Cr | Mo |
|---|---|---|---|---|---|---|---|
| A | 0.35 | 0.29 | 0.69 | 0.008 | 0.004 | 0.97 | 0.18 |
| B | 0.35 | 0.22 | 0.75 | 0.007 | – | 1.01 | 0.23 |
A flat plate specimen of 70 × 35 × 1.6 mm was cut from the steel B and hydrogen-charged using the atmospheric corrosion test. In addition, a U-bend specimen, which was created using a bending process used for automobile parts, was also subjected to an atmospheric corrosion test. To prepare the U-bend specimen, a 150 × 30 × 1.6 mm specimen was cut from steel A, and the long side end face was milled to eliminate the effect of shearing strain due to shear cutting. This specimen was bent into a U shape by 3-point bending, and the bending radius was 10 mm. The load stress is the value obtained by multiplying the amount of strain measured using a strain gauge attached to the top of the bend by Young’s modulus. The stress of 1500 MPa is applied to the bent portion by tightening the bolt passed through the specimen with a nut. An atmospheric corrosion test was conducted at Choshi in the Japan Weathering Test Center20) for the purpose of hydrogen charging due to corrosion. The duration of the atmospheric corrosion test was 2 weeks for the flat specimens and 3 months for the U-bend specimens. After the atmospheric corrosion test, the specimens were stored in a cooled state with dry ice and liquid nitrogen to suppress the escape of hydrogen that entered the steels. The specimen for hydrogen analysis was cut from the corroded U-bend specimen. The cutting was conducted in the shortest possible time to prevent the hydrogen from escaping, and the specimen was cooled with liquid nitrogen before and after cutting.
A flat plate specimen of 10 × 10 × 1.6 mm was cut from the steel B and hydrogen-charged by cathodic charging test. A potentiostat/galvanostat was used for the constant current controlled cathodic charging test. A platinum was used as the counter electrode, and 0.1 M NaOH aqueous solution was used as the solution. The cathodic charging test was performed at around 20°C, and the cathodic current density and charging time were 1 μA mm−2 and 48 hours, respectively.
2.3. BlastingThe rust formed by atmospheric corrosion was removed using blasting. A handy blaster (SNM Asia Co., Ltd.) was used as the blasting device, and compressed air or compressed dry air was used as the air source. A silica abrasive (No. 9, Takeori Suisan Co., Ltd.) and alumina abrasive (WHITE ABRAX F220, Saint-Gobain Co., Ltd.) were used for blasting. Although all the silica abrasives are the same product, the hydrogen analysis results changed depending on the lot, so the silica abrasives A, B, and C were distinguished for each lot. In order to prevent hydrogen from escaping due to the temperature rise, cooling with liquid nitrogen was conducted before and after the blasting. The same blasting was performed for the non-corroded specimens and the hydrogen-charged specimens by cathodic charging test to investigate the effect of blasting on hydrogen analysis. The amount of water adhering to silica sand and alumina used for the abrasives was evaluated by using the heat vaporization Karl Fischer method up to 105°C.
2.4. Hydrogen Content Measurement and Visualization of Local Hydrogen DistributionThe hydrogen content was evaluated with an atmospheric pressure ionization mass spectrometer (API-MS).21) The diffusible hydrogen that affects hydrogen embrittlement is generally released up to 300°C. Therefore, the hydrogen content was evaluated as an integrated value of hydrogen released when the temperature was raised from room temperature to 300°C at a heating rate of 12°C min−1.
The visualization of local hydrogen distribution was evaluated by SIMS. Here, deuterium (D) with low isotope abundance ratio was used instead of hydrogen (H) as a tracer. With the isotope labeling method using D as a tracer, the D that entered steel forms an environment that can be evaluated separately from the H originally present in steel. Additionally, since it is possible to distinguish the D from the hydrogen background resulting from the naturally existing water (H2O), the evacuation time for reducing the hydrogen derived from the background can be reduced. This makes it possible to start the SIMS evaluation before the hydrogen desorbs in the vacuum chamber.22) The negative deuterium ions (2D−) were obtained in the scanning ion image mode under the primary ion beam conditions of Cs+ and 15 keV.
In order to understand the effect of rust on the hydrogen analysis in steel, the corroded steel after the atmospheric corrosion test was intentionally blasted three times for a short time of less than 1 s so that some rust on the specimen remained. The results of the hydrogen analysis are shown in Fig. 1. Here, silica A was used for the blast abrasive. As shown in Fig. 1(a), the rust formed in the atmospheric corrosion test was partially removed by blasting, but some remained. Figure 1(b) shows the hydrogen contents of the specimens with residual rust. In addition to the hydrogen desorption on the low temperature side with a peak near 120°C, hydrogen release on the high temperature side with a peak around 250°C was observed. The hydrogen desorption peaking around 120°C is diffusible hydrogen, and part of it may be derived from hydrogen that entered the steel during the atmospheric corrosion test. On the other hand, the hydrogen desorption peak around 250°C is assumed to be derived from rust. Ishiguro et al. investigated in detail the hydrogen desorption behavior when analyzing the steels with rust by a thermal-desorption-spectroscopy-based gas chromatograph, and the reaction between rust and iron released hydrogen in a high temperature range of 300–400°C.19) Similarly, in this experiment, we assumed that the rust and steel reacted during the thermal desorption analysis and hydrogen was released on the high temperature side. The hydrogen desorption on the high temperature side extends to the low temperature side and hinders the quantification of diffusible hydrogen in the steel on the low temperature side, which is likely to affect hydrogen embrittlement. Therefore, the rust acts as an inhibitor for the precise measurement of the hydrogen content in steel and must be sufficiently removed before the hydrogen analysis. Next, we examined the removal of rust by blasting.

(a) Photographic images of specimen before and after insufficient blasting using silica A to remove the rust formed by atmospheric corrosion. (b) Hydrogen desorption profile of specimen with residual rust after insufficient blasting. (Online version in color.)
To investigate the effect of the blasting on the hydrogen analysis, a lengthy blasting was performed so that the rust on the specimen after atmospheric corrosion test was sufficiently removed. Here, silica sand A is used for the abrasive, and in order to investigate the effect of the blasting time, the number of blasting is changed by setting the blasting time to 1 s, 3 s, 10 s, 30 s, and the total time was fixed at 30 s. For example, if the blasting time is 1 s, the blasting is conducted for 1 s and then cooling with liquid nitrogen is performed 15 times on each of the front and back surfaces of the specimen, for a total of 30 times (30 s). The results obtained are shown in Fig. 2. The rust formed by atmospheric corrosion is almost completely removed by blasting (Fig. 2(a)). Therefore, the following discussion will proceed assuming that rust has almost no effect on hydrogen analysis. As shown in Fig. 2(b), the hydrogen content increases as the blasting time per blast increases. Figure 2(c) shows that the longer the blasting time, the greater the hydrogen release, and the more remarkable the hydrogen release on the high temperature side, which does not completely desorb even when the temperature rises to 300°C. We expected at first that the longer the blasting time, the higher the temperature of the specimen and the lower the hydrogen content due to the heat generated by the blasting, but this result was the opposite of what we expected. Actually, it was confirmed that the surface temperature increased to −22°C, −19°C, −4°C, and 25°C as the blasting time per blast time increased to 1 s, 3 s, 10 s, and 30 s by a surface thermometer measurement of the specimen immediately after blasting. The hydrogen, which entered the steel due to corrosion, is thought to be more likely to escape as the temperature of the specimen rises. Therefore, the increase of hydrogen content due to the prolonged blasting time cannot be explained by the hydrogen that entered in the atmospheric corrosion test. That is, the blasting itself affects the measurement of the hydrogen content.

(a) Photographic image of specimen after sufficient blasting using silica A to remove the rust formed by atmospheric corrosion. (b) Hydrogen contents and (c) hydrogen desorption profiles of corroded specimens after blasting when changing the time per blasting with the total time of blasting as 30 seconds. (Online version in color.)
In order to precisely evaluate the hydrogen content in the steel due to corrosion, it is necessary to remove rust and suppress the hydrogen entry due to blasting. For the purpose of suppressing the hydrogen entry due to blasting, we investigated the hydrogen source. Figure 5 shows the hypothesis of the hydrogen entry mechanism into steel. We hypothesized a model in which the water that could be a hydrogen source is reduced by the fresh steel surface exposed during the blasting to generate hydrogen, and a part of the hydrogen enters the steel. As the hydrogen sources, the effects of (i) frost adhering to the surface of the specimen taken out from liquid nitrogen, (ii) water contained in the air source used for blasting, (iii) water in abrasives, and (iv) water in the atmosphere were investigated below.

Schematic illustration of (i) frost, (ii) blasting air, (iii) water in abrasive, and (iv) atmosphere, which are assumed to be hydrogen sources for increasing the hydrogen content due to blasting. (Online version in color.)
Both the frosted specimen taken out from liquid nitrogen and the specimen without frost were blasted. The silica C was used for the blasting, and the front and back surfaces of the specimens were blasted for 15 s each, for a total of 30 s. The hydrogen contents were 0.08 ppm and 0.09 ppm for the specimens with and without frost, showing no significant difference. Therefore, the frost is not a hydrogen source for increasing hydrogen content due to blasting.
3.2.2. Effect of Water Contained in the Air SourceTo investigate the effect of water contained in the air source, a comparison was made when compressed air and compressed dry air were used as the air source. Silica B was used for the abrasive, and the front and back surfaces of the test piece were blasted for 15 s each, for a total of 30 s. The hydrogen content when using compressed air was 0.13 ppm, and the hydrogen content when using compressed dry air was 0.14 ppm. Therefore, the water contained in the air source was not a hydrogen source.
3.2.3. Effect of Water Contained in the AbrasiveIn order to investigate the influence of the water contained in the abrasive, the change when the abrasive was dried to remove the water was investigated. The silica B used for the abrasive was dried at 200°C × 24 h or more. After the drying process, both the dried silica and the molecular sieve were stored for several hours in a desiccator that was dehydrated with a molecular sieve in advance to prevent the moisture in the atmosphere from reattaching to the silica as it cools to room temperature. The amount of water contained in the silica, which was evaluated by the Karl Fischer method, was 0.12 mass% before the drying treatment and decreased to 0.07 mass% by the drying treatment. Figure 6(a) shows the results of thermal desorption analysis of the specimen that had been blasted with dried silica for 15 s each on the front and back surfaces for a total of 30 s. The hydrogen content of specimen blasted by abrasive without the drying treatment was 0.14 ppm, whereas the hydrogen content decreased to 0.11 ppm when the dried silica was used. The hydrogen content decreased as the amount of water contained in the silica decreased, indicating the water contained in the abrasive could be a hydrogen source. To directly prove that the water contained in the abrasive acts as a hydrogen source, blasting was performed using silica to which deuterium water is attached, and it was verified whether deuterium was detected in the specimen after the blasting. Compared to light water (H2O), deuterium water (D2O) has an extremely low abundance ratio in nature, so it can be concluded that the water contained in the abrasive grains is the hydrogen source when deuterium is detected in a specimen blasted using abrasive with deuterium water. The silica after drying treatment was stored with deuterium water in a beaker in a desiccator dehydrated in advance with a molecular sieve for several hours and cooled to room temperature. By using this treatment (hereinafter referred to as deuterium water substitution treatment), deuterium water evaporated from the beaker can be adsorbed on the silica instead of the light water desorbed by the drying treatment. The silica with deuterium water was used to blast the front and back surfaces of the specimen for 15 s each, for a total of 30 s. Figure 6(b) shows the deuterium desorption profiles of specimens after blasting. When the deuterium water substitution treatment was not performed, almost no deuterium was detected in the specimen after the blast treatment regardless of whether or not the silica was dried. Remarkably, deuterium was detected only in the specimen blasted using the silica subjected to the deuterium water substitution treatment. This result demonstrated that the water contained in the abrasive acts as a hydrogen source for increasing hydrogen content of steel due to the blasting. As shown in Fig. 6(a), the hydrogen content after deuterium water substitution treatment was 0.09 ppm, which was lower than the content without drying treatment (0.14 ppm) and the content after drying treatment without deuterium water substitution treatment (0.11 ppm). This is because the amount of light water contained in the silica sand decreased due to the drying treatment and the suppression of reattachment of the light water due to the deuterium water adhering to the abrasive grains.

(a) Hydrogen and (b) deuterium desorption profiles of non-corroded specimens after blasting using abrasive without baking, with baking, and with baking and deuterium substitution. Silica B was used as abrasive. (Online version in color.)
To control the amount of water in the atmosphere, blasting was performed here in a glove box. By using silica sand C whose water content in the abrasive was reduced by the drying process described in section 3.2.3, the specimens were blasted inside the glove box dried by a molecular sieve (relative humidity: 21%) and the undried glove box (relative humidity: 55%). Here, the blasting time was 15 s for each of the front and back surfaces of the specimen, for a total of 30 s. The hydrogen content when using the undried glove box was 0.06 ppm, and the hydrogen content when using the dried glove box was 0.05 ppm, showing no significant difference. Therefore, it was confirmed that the effect of water in the atmosphere was small.
From the above results, it was clarified that the hydrogen source for increasing hydrogen content by the blasting is mainly the water contained in the abrasives.
3.3. Suppression Method of Hydrogen Entry into by BlastingFor suppressing the increase in the hydrogen content due to the blasting, blasting using abrasives with a small amount of adhering water was examined. Specifically, blasting was performed using alumina in addition to silica as abrasives. The water content of silica A, B, and C was 0.07, 0.12, 0.09 mass%, and although they all had the same product name, the amount of water differed from lot to lot. Although the cause of this is not clear, we estimated that the amount of adhering water differs depending on the manufacturing method, manufacturing time, or storage method. The water content of alumina was 0.02 mass%, which was less than that of silica. Figure 7(a) shows the hydrogen content measurement results of the specimens that were blasted using these abrasive grains. The number of blasting was changed by setting the blasting time to 1 s, 3 s, 10 s, 30 s, and the total time was fixed at 30 s. The hydrogen content tended to decrease as the time per blasting became shorter for all abrasive. By using alumina, the hydrogen content became lower than that of silica. Figure 7(b) shows the relationship between the hydrogen contents of specimens after blasting and the water contents of abrasives. The hydrogen content increases as the amount of water contained in the abrasive increases, regardless of whether the blasting time is 1 s or 30 s. This result also shows that the amount of water adhering to the abrasives is a hydrogen source and causes an increase in the hydrogen content by blasting. To suppress the increase in the hydrogen content due to blasting, it is effective to shorten the blasting time per time and to use abrasives with a small amount of adhering water.

(a) Relationship between hydrogen contents of non-corroded specimens after blasting and time per blasting when changing the abrasives. (b) Relationship between hydrogen contents of non-corroded specimen after blasting and water contents of abrasives. (Online version in color.)
As shown in Fig. 5, it was assumed that the fresh surface of the steel exposed by blasting reacts with the water (particularly water contained in abrasives), which results in hydrogen generation and entry into steel. Here, we consider the details of the hydrogen entry mechanism into steel by blasting, using the analysis of the hydrogen desorption profile and the hydrogen visualization by SIMS. The hydrogen desorption profile of the specimen after blasting consists of two types of hydrogen release: hydrogen release on the low temperature side, which has a peak in the 100 to 200°C range, and hydrogen release on the high temperature side, which does not decrease even at 300°C (Fig. 3). To clarify whether these hydrogens are diffusible hydrogen or non-diffusible hydrogen that has entered the steel, the hydrogen contents of the specimens were evaluated after leaving at room temperature for a certain period after the blasting as shown in Fig. 8. The hydrogen content the specimens stored in liquid nitrogen immediately after blasting was 0.18 ppm. On the other hand, the hydrogen content decreased to 0.11 ppm after 1 day and remained almost constant for up to 10 days. The hydrogen desorption profiles (Fig. 8(b)) show that the hydrogen release on the low temperature side, which has a peak in the 100 to 200°C range, disappears after being left at room temperature, suggesting that it is diffusible hydrogen. On the other hand, the hydrogen desorption on the high temperature side remains regardless of the leaving time, indicating that it is non-diffusible hydrogen. Generally, the hydrogen charged by corrosion and/or the cathodic charging test exists in steel as diffusible hydrogen and escapes while left at room temperature. The hydrogen release on the low temperature side of the peak in the 100 to 200°C range also escapes when left at room temperature, suggesting that it is diffusible hydrogen that entered the steel.

(a) Hydrogen contents and (b) hydrogen desorption profiles of non-corroded specimens after blasting using silica A, B, C when changing the time per blasting with a total time of blasting of 30 seconds. (Online version in color.)

(a) Hydrogen contents and (b) hydrogen desorption profiles of non-corroded specimens after blasting using silica C when changing the thickness of specimens. (Online version in color.)
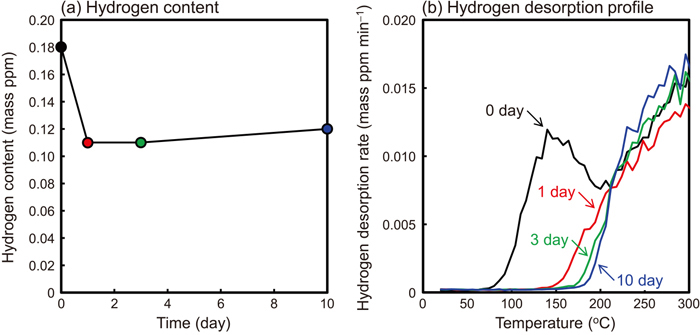
Time dependence of (a) hydrogen contents and (b) hydrogen desorption profiles of non-corroded specimens kept at room temperature after blasting. Silica B was used as abrasive. (Online version in color.)
To directly prove that hydrogen enters the steel by blasting, SIMS was used to visualize the hydrogen in the steel cross section. Figure 9 shows the results of visualization of deuterium in the cross section of the specimen that was blasted using abrasives that were replaced with deuterium water after the drying treatment as in Section 3.2.3. The region where 72FeO− is strongly detected in Fig. 9(c) is the cross-sectional part of the steel, and it can be seen from Fig. 9(a) that deuterium (2D−) exists inside the steel. This result clearly shows that hydrogen derived from water contained in the abrasives enters the steel. The deuterium exists not only inside the steel but also on the surface of the steel. The location of deuterium on the steel surface and the location of silicon (28Si−) coincided. Since the detected silicon may derive from silica remaining on the steel surface, the cross-sectional SEM observation and EDX analysis of the specimen before and after the blasting were conducted as shown in Fig. 10. The number of blasting was changed by setting the blasting time to 1 s and 30 s, and the total time was fixed at 30 s. Si and O, which were not observed before the blasting (Fig. 10(a)), were confirmed on the surface of the specimen after the blasting, indicating that silica remained on the steel surface. Therefore, the deuterium on the steel surface observed by SIMS was derived from the deuterium water contained in the remaining silica. The longer the blast time (compare 30 s in Fig. 10(c) with 1 s in Fig 10(b)), the greater the amount of silica remained on the steel surface. As described above, two states of hydrogen were confirmed by SIMS; hydrogen existing in the steel and hydrogen derived from water contained in the abrasives remaining on the steel surface. Considering the correspondence between this and the two types of hydrogen release seen in the hydrogen desorption profile, the diffusible hydrogen which entered the steel is the hydrogen which is released on the low temperature side in the 100 to 200°C range. And the water contained in the abrasives on the steel surface which reacts with the steel during the temperature desorption analysis is the cause of the hydrogen release on the high temperature side. Since the water contained in the abrasives remaining on the steel surface is stable at room temperature, it can be explained that hydrogen release was observed on the high temperature side without changing even in the specimen left at room temperature after the blasting.

SIMS images of (a) 2D−, (b) 28Si−, and (c) 72FeO− in specimen after blasting using abrasive with baking and deuterium substitution. Silica B was used as abrasive. (Online version in color.)

SEM and EDX mapping images of specimens (a) before blasting, (b) after blasting with 1 s time per blasting, and (c) after blasting with 30 s time per blasting. Silica B was used as abrasive. (Online version in color.)
Based on the above results, the mechanism of hydrogen entry into steel by blasting is proposed as shown in Fig. 11. The fresh surface of steel exposed by the blasting reduces the water contained in the abrasives to generate hydrogen, and a part of it enters the steel as diffusible hydrogen (Fig. 11(b)). After the blasting, diffusible hydrogen exists in the steel, and abrasives containing water remain on the steel surface (Fig. 11(c)). During the thermal desorption analysis, the diffusible hydrogen in the steel is released with a peak on the low temperature side in the range of 100 to 200°C. When the temperature is raised, the water contained in the abrasives on the steel surface reacts with the steel (Fe + H2O → Ferrioxide + H2), and hydrogen is generated on the high temperature side (Fig. 11(d)).

Schematic illustration of hydrogen entry mechanism into steel by blasting. (Online version in color.)
Shortening the blasting time and using abrasives with a small amount of adhering water are effective in suppressing the increase in hydrogen content of steel due to blasting as shown in Fig. 7. Here, the suppression mechanism is considered. The mechanism of suppression in hydrogen entry by shortening the blasting time is thought to be due to the suppression in the temperature rise of the steel due to heat generated by the blasting and the reduction of amount of abrasives remaining on the steel. Generally, the redox reaction (corrosion reaction) between steel and water is accelerated as the temperature rises. Therefore, when the blast time is long, the corrosion reaction between steel and water of abrasives becomes active due to the temperature rise, so that the hydrogen generation reaction and hydrogen entry are promoted. On the other hand, when the blasting time per time is short and the temperature of the steel is low, we assumed that the corrosion reaction and the hydrogen generation and entry reaction were suppressed. In addition, as shown in Fig. 10, the shorter the blasting time, the smaller the amount of abrasives remaining on the steel surface. This is because the longer the blasting time, the more stable the air pressure and the stronger the pressure at which the abrasives collide with the steel. Assuming that the hydrogen desorption on the high temperature side is caused by the reaction between the water contained in the abrasives and the steel, the shortening of the blast time decreases abrasives in the steel surface, which results in the decrease of hydrogen desorption on the high temperature side.
The suppression in hydrogen entry by using abrasives with a small amount of water is understood because the cause of hydrogen desorption on the low temperature side and the high temperature side is the water contained in the abrasives. Namely, as the amount of water adhering to the abrasives is smaller, the hydrogen content generation by the reaction with the fresh surface of steel and hydrogen entry are reduced, and the diffusible hydrogen content on the low temperature side is reduced. In addition, since the amount of water in the abrasives remaining on the steel surface after the blasting is reduced, the hydrogen desorption on the high temperature side generated by the reaction with the steel during the temperature desorption analysis is also reduced.
4.3. Blasting Method to Correctly Evaluate Hydrogen Content of SteelThe method of suppressing the increase in the hydrogen content due to the blasting was examined as described above, but it is also possible that hydrogen in the steel escapes due to heat generation during the blasting. Therefore, we investigated whether the blasting promote the escape of hydrogen in steel. The hydrogen-charged specimen by cathodic charging test was blasted and subjected to hydrogen analysis. As a comparative example of inappropriate blasting, Fig. 12(a) shows the hydrogen desorption profiles when silica B with a high-water content was used for the abrasive, and the front and back surfaces of the specimen were blasted for 15 s each. For the hydrogen-charged specimen without blasting, the desorption of diffusible hydrogen (0.04 ppm) with a peak on the low temperature side in the 100 to 200°C range was observed. On the other hand, for the specimen blasted after cathodic charging, the hydrogen desorption on the low temperature side in the range of 100 to 200°C and the high temperature side up to 300°C were detected, and the hydrogen content increased to 0.20 ppm. The increase in hydrogen content (0.14 ppm) was observed for the hydrogen non-charged specimen after blasting. As a result of the hydrogen entry into the steel by the blasting after the cathodic charging, the hydrogen content increased from 0.04 ppm before the blasting to 0.20 ppm. Figure 12(b) shows the hydrogen desorption profile of specimen blasted using silica B with 1 s time per blasting (the total time was fixed at 30 s). By shortening the blasting time from 30 s to 1 s, the increase in hydrogen content due to blasting was reduced from 0.14 ppm to 0.04 ppm, and the hydrogen content blasted after cathodic charging also decreased from 0.20 ppm to 0.10 ppm. However, in the case of using abrasives with a high amount of adhering water such as silica, the hydrogen content of steel could not be measured correctly due to hydrogen entry by blasting even if the blasting time was shortened. On the other hand, Fig. 12(c) shows the hydrogen desorption profiles when alumina with a small amount of water was used for the abrasive, and the front and back surfaces of the specimen were blasted for 1 s time per blasting (the total time was fixed at 30 s). The increase in hydrogen due to blasting was only 0.01 ppm. In addition, the hydrogen desorption profile of the hydrogen-charged specimens did not change significantly before and after the blasting. Thus, the developed method can suppress both hydrogen entry and hydrogen escape during blasting, and it is possible to accurately measure the hydrogen content of steel.

Hydrogen contents and hydrogen desorption profiles of non-corroded specimens with blasting, without blasting after charging, and with blasting after charging. (a) Silica B was used as abrasive with 30 s time per blasting. (b) Silica B was used as abrasive with 1 s time per blasting. (c) Alumina was used as abrasive with 1 s time per blasting. (Online version in color.)
Finally, the hydrogen contents of the corroded U-bending test piece were compared when the rust was removed by different blasting methods. Two specimens for hydrogen analysis were cut out from the top of the U-bending steel after the atmospheric corrosion test, and rust was removed by changing the blasting method. As a comparative example of inappropriate blasting, Fig. 13(a) shows the hydrogen desorption profiles when silica B with a high-water content was used for the abrasive, and the front and back surfaces of the specimen were blasted for 15 s each. As a suitable blasting method, Fig. 13(b) shows the hydrogen desorption profiles when alumina with a low-water content was used for the abrasive, and the front and back surfaces of the specimen were blasted for 1 s each. A photograph of the appearance of the hydrogen analysis specimen cut out from the U-bending steel is also shown, and no rust remained after the blasting. The hydrogen content when silica sand was used was 0.23 ppm, but this is due to the increase in the hydrogen content (0.18 ppm) by blasting. On the other hand, the hydrogen content is 0.05 ppm when alumina is used. This is the true hydrogen content which entered the steel by corrosion because the increase and escape of hydrogen due to the blasting is negligible under these conditions. Compared to the peak in the hydrogen desorption profile of specimen without bending (Fig. 12), the peak from the specimen with bending is shifted from approximately 150°C to 200°C. This is because hydrogen was trapped on the more stable high temperature side due to lattice defects such as dislocations and vacancies introduced by U bending, and/or the diffusion rate of hydrogen decreased, which resulted in the delayed hydrogen desorption. The appearance after blasting is the same as when silica sand and alumina are used for the abrasives, but the blasting method for removing rust significantly affects the hydrogen content. In the case of the inappropriate blasting, the hydrogen content entering from the environment is evaluated excessively due to the increase in the hydrogen content accompanying the blasting. If the hydrogen content entering from the environment is overestimated, there is a problem that the applicability of high-strength steel may be misunderstood in some cases because the usage environment is harsher than the actual situation. This research provides a hydrogen evaluation method for precisely measuring the hydrogen content of corroded steel, and is expected to be utilized for hydrogen embrittlement research of high-strength steel, which is expected to develop further in the future.

Hydrogen contents and hydrogen desorption profiles of the corroded U bend specimen after blasting and the non-corroded specimen after blasting. (a) Silica B and (b) alumina were used as abrasives. (Online version in color.)
In this study, the effect of blasting to remove rust on hydrogen content measurement was examined for the purpose of establishing a method for correctly evaluating hydrogen that has entered steel in a corrosive environment. The following conclusions have been obtained.
(1) The phenomenon of hydrogen entry into steel by blasting was demonstrated for the first time. It should be noted that the effect is remarkable for specimens with a large specific surface area. The blasting becomes an inhibitor in the accurate measurement of the hydrogen content of steel.
(2) The hydrogen source for increasing the hydrogen content due to the blasting is mainly the water contained in the abrasives.
(3) The mechanism of increasing the hydrogen content by blasting is that hydrogen is generated and enters the steel due to the reaction between the fresh surface of steel exposed by blasting and the water contained in the abrasives. Additionally, the water in the abrasives remaining on the steel surface reacts with steel during the thermal desorption analysis to release hydrogen.
(4) To suppress hydrogen entry due to the blasting, it is effective to use abrasives with a small amount of water and to remove rust by repeating a short blasting time in order to suppress the temperature rise of specimens.
This article is based on results obtained from Innovative Structural Materials Association (ISMA) commissioned by the New Energy and Industrial Technology Development Organization (NEDO).