2022 年 62 巻 9 号 p. 1827-1835
2022 年 62 巻 9 号 p. 1827-1835
In this study, the in situ compressive strength of iron coke in high-temperature carbonization was investigated. The high-temperature compressive strengths of iron coke briquettes with 20, 30, and 40% iron ore with carbonization at 700, 800, 900, and 1000°C for 3 h were systematically studied. The mass loss and microstructure of the iron coke during carbonization were also investigated. The results showed that the mass loss increased with the iron ore content from 20 to 40%. The liquid phase content of the slag in iron coke was calculated using FactSage software. Results showed an increased of liquid phase slag content with increasing of carbonization temperature and decreasing of Fe3O4 content. Coal coking, iron oxide reduction, pitch volatilization, and CO2 gasification in the carbonization process could significantly change the micromorphology of iron coke and influence its high-temperature compressive strength. At a carbonization temperature of 900°C, the mass loss of iron coke increased from 27.59 to 30.23% and the high-temperature compressive strength decreased from more than 4200 to 1589 N with an increase in the iron ore content from 20 to 40%. The degradation mechanism of the high-temperature compressive strength of iron coke with 30 and 40% iron ore, and increased carbonization temperature from 800 to 1000°C, was discussed.
The iron and steel industry is a pillar of global economic and social development around the world.1) Because the iron and steel industry is energy- and emission-intensive, its associated energy and environmental problems are receiving increased attention. Approximately 5% of the global energy is consumed by the iron and steel industry, and blast furnace (BF) ironmaking processes account for 75% of the total energy consumption of iron and steel plants.2,3) Therefore, ironmaking has the greatest carbon reduction potential in steel production. It can be predicted that BFs will remain the dominant ironmaking equipment in China for the foreseeable future.4,5) Using a low-carbon ironmaking charge in BFs to reduce carbon emissions is currently a popular research topic.6,7,8) Iron coke, also known as ferro-coke and iron-carbon agglomerates, is a type of coke with high reactivity. It is produced by blending iron ore with coal during coke production.8) Iron coke can be used in BFs to decrease the gasification starting and thermal reserve zone temperatures, save energy, and reduce carbon emissions.9,10,11,12) The high-temperature compressive strength properties of iron coke are the basis for its successful preparation in carbonization furnaces and further application in BFs. A suitable high-temperature compressive strength can mitigate some of the operational problems of BFs, such as reduced permeability in the shaft and hearth areas; undesirable gas and temperature distribution; and the possibility of hanging the burden.13,14) Therefore, high-temperature compressive strength is one of the most important indices of iron coke.
Recent studies have focused on the compressive strength and high-temperature compressive strength of coke. Ono et al.15) evaluated the strength reduction during the reaction of the coke with CO2 or H2O, and Saito and Tsukamoto16) measured the compression stress during the reaction of CO2 at high temperatures. Nishioka et al.17) believed that the coke strength is determined by the microstrength, which is considered to reflect the strength of the coke matrix. Holowarty and Squarcy et al.18) conducted a compression test of BF coke under a N2 atmosphere at approximately 1649°C and found that the compression strength indices of all cokes were higher at 1649°C than at room temperature. The findings also suggested that the volatile matter content; ash content, composition, and fusion point; coke cell wall porosity and thickness; and internal cracking and fissuring within the coke body appreciably affect the coke characteristics. Juho et al.19) investigated and evaluated the hot strength of coke and showed that the mean ultimate strength of coke decreased from 15.1 to 12.1 MPa when heated from 1000 to 1600°C. Varon Cardona et al.20) investigated coal–charcoal composite biocoke as a sustainable alternative for ironmaking and found that the bulk density and compressive strength increased with increased wood charcoal in briquettes. In contrast, these properties deteriorated with wood charcoal weight percentages higher than 15 wt.%. Grant et al.21) believed that higher-strength cokes have smaller pore walls and a larger number of pores (with smaller pore sizes) than weaker cokes (fewer pores, larger cell walls, and larger pores). Lan et al.22) found that the presence of H2 aggravates the increase in coke porosity in the low-temperature region but reduces the internal porosity in the high-temperature region, improving the strength of coke after a high-temperature reaction. Yamaoka and Suyama et al.23) believed that coke is a compound possessing multiple types of coke textures with respective strengths and reactivities. Both, the strength and reactivity of coke, are dominated by the coke texture composition and pore volume fraction. Thus, research has provided a deep understanding of the high-temperature compressive strength of coke.
Some studies have focused on the strength of iron coke. Uchida et al.24) investigated the effect of iron ore reduction on the strength of ferro-coke with hyper-coal addition; they found that the strength of ferro-coke increased with the blending ratio of hyper-coal, and was determined not by the “defect” around iron oxide, but by the wall thickness. Wang et al.25) found that with an increase in the iron ore addition ratio, the compressive strength of an iron coke hot briquette initially increased, followed by a decrease; the compressive strength reached a high value of 3490.89 N with 15% iron ore addition. Yin et al.9) found that Fe2O3 degrades the strength of iron coke by decreasing the aromaticity, while the average stacking height increases the interlayer spacing of the crystallite, aliphatic chain length, and hydrocarbon-generating potential of mixed coal. Xu et al.26) found that the type and amount of iron ore significantly influence the drum and compressive strengths of ferro-coke, whose strength decreased with an increase in the iron content. In summary, the cold strength of iron coke has been comprehensively studied. However, few studies have focused on the high-temperature compressive strength of iron coke, which is of practical significance for high-temperature carbonization in carbonization furnaces and subsequent application in BFs. In this study, the high-temperature compressive strength of iron coke was investigated under various carbonization conditions.
In this study, the high-temperature compressive strengths of iron coke briquettes with 20, 30, and 40% iron ore with carbonization temperatures of 700, 800, 900, and 1000°C were systematically studied. The mass loss and microstructure of the iron coke during carbonization were investigated to elucidate the mechanism of the in situ compressive strength variation of iron coke with varying iron ore content and carbonization temperature.
The raw materials used in preparing the iron coke include one type of iron ore, four types of coals, and one type of soft pitch, all of which were supplied by HBIS Group, China. The chemical composition of the iron ore was determined using chemical composition analysis and is listed in Table 1. The total iron (TFe), FeO, and SiO2 contents in the iron ore were 65.36, 21.86, and 6.35 wt.%, respectively. As the raw coal materials, 1/3 coking coal, prime coking coal, lean coal, and anthracite were selected. The proximate and coking property analyses of the four coal fines were performed by the National Quality Control and Inspection Center for Coking and Chemical Products of Metallurgy, China, and the results are listed in Table 2. Before briquette preparation, the iron ore was ground to maintain a particle size of < 75 μm, accounting for approximately 80% of the sample. Additionally, all the coals were crushed and screened to < 4 mm. Soft pitch (additional 5 wt.%) was used as the binder in briquette preparation. The ingredients of the raw briquette are listed in Table 3.
| TFe | FeO | CaO | SiO2 | MgO | Al2O3 |
|---|---|---|---|---|---|
| 65.36 | 21.86 | 0.17 | 6.35 | 0.45 | 0.45 |
| Coal | Fixed carbon (%) | Ash (%) | Volatile matter (%) | Chemical composition of ash, wt (%) | Caking index, G (%) | Thickness of adhesive layer, Y (mm) | |||
|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | MgO | Al2O3 | ||||||
| 1/3 coking coal | 61.21 | 8.96 | 29.08 | 4.02 | 49.78 | 0.78 | 38.84 | 74 | 15.0 |
| Prime coking coal | 69.84 | 10.74 | 19.10 | 2.23 | 51.96 | 0.56 | 37.90 | 75 | 14.0 |
| Lean coal | 76.50 | 9.90 | 13.16 | 2.12 | 49.29 | 0.40 | 37.37 | 15 | N/A |
| Anthracite | 78.25 | 13.36 | 7.30 | 3.59 | 50.30 | 1.42 | 35.70 | N/A | N/A |
| No. | Iron ore | 1/3 coking coal | Prime coking coal | Lean coal | Anthracite | Soft pitch |
|---|---|---|---|---|---|---|
| 1 | 20 | 51.5 | 11.4 | 11.4 | 5.7 | 5 |
| 2 | 30 | 45 | 10 | 10 | 5 | 5 |
| 3 | 40 | 38.5 | 8.6 | 8.6 | 4.3 | 5 |
The experimental process used in this study is illustrated in Fig. 1. The iron ore fines and coals were first dried at 105°C for 5 h. Subsequently, the dried iron ore and coal fines with varying contents (Table 3) were thoroughly mixed in a planetary mixer (Changsha Tianchuang Powder Technology Co., Ltd, QXQM-4L type) for 10 min. Finally, soft liquid pitch (5 wt.%), preheated in a resistance wire heating furnace, was mixed with the iron ore-coal mixed material and stirred to obtain the final raw mixture material. When the mixture material was prepared, it was pressed at 60°C using roller briquetting equipment (Luoyang Kaizheng Environmental Protection Equipment Co., Ltd., KYS175 type) to prepare briquettes (ellipsoid, external size: 31.8×26.6×17.3 mm) under a linear pressure of 29.4 kN cm–1. Iron coke can be obtained by carbonizing briquettes in an inert atmosphere at a high temperature.

Flowchart of the experimental process. (Online version in color.)
The experiments for determining the high-temperature compressive strength of iron coke were conducted in a MoSi2 furnace, and the apparatus is shown in Fig. 2. The briquettes were placed in a high-purity graphite crucible with an inner diameter and height of 73 and 62 mm, respectively, and a bottom with a uniform pore arrangement to facilitate the passage of gas. A high-purity graphite crucible with loaded briquettes was placed in the constant-temperature zone of the MoSi2 furnace under a N2 atmosphere (99.99%, 5 L min−1). The heating rate of the furnace was 10°C min−1 up to 900°C and 5°C min−1 above 900°C. The carbonization temperatures were set to 700, 800, 900, and 1000°C, and the carbonization time was set to 3 h once the temperature was reached. The graphite rod was then lowered to the sample to evaluate its high-temperature compressive strength. The pressure sensor recorded the maximum compressive strength corresponding to the pressure of the broken iron coke. This value was obtained from the panel, and the in situ compressive strength of iron coke after carbonization could be characterized. The entire experiment was conducted in the furnace under a N2 atmosphere. The mass loss of the briquette during carbonization was also investigated. The iron coke surface was analyzed using an optical microscope (OM).

Schematic of the high-temperature compressive strength apparatus: (1) pressure sensor, (2) tail gas output, (3) graphite rod, (4) iron coke, (5) graphite crucible, (6) corundum ball, (7) thermocouple. (Online version in color.)
The variation in the mass loss of the iron coke during carbonization at varying carbonization temperatures is shown in Fig. 3. The mass of the iron coke was lower than that of the raw briquette because of coal coking, iron oxide reduction, pitch volatilization, and CO2 gasification during carbonization. The mass loss increased with increasing carbonization temperature, indicating that carbonization can be promoted by increasing the carbonization temperature. In addition, the mass loss increased with the iron ore content from 20 to 40%, indicating that it increased with decreasing coal content. This suggests that the mass loss contribution of iron oxide reduction and CO2 gasification was greater than that of coking.

Variation in the mass loss of the iron coke with varying carbonized temperatures, using (A) 20, (B) 30, and (C) 40% iron ore. (Online version in color.)
The variation in the high-temperature compressive strengths of iron coke at varying carbonization temperatures is shown in Fig. 4. The compressive strength of the iron coke was higher than that of the briquette, indicating that carbonization can increase the compressive strength. With 20% iron ore, the high-temperature compressive strength of the iron coke increased from 1815 N to more than 4200 N as the carbonization temperature increased from 700 to 1000°C because of coal coking. With 30 and 40% iron ore, the high-temperature compressive strength initially increased and then decreased, as the carbonization temperature of iron coke increased from 700 to 1000°C. A high content of iron ore in iron coke is not beneficial for obtaining high-temperature compressive strengths at carbonization temperatures above 800°C. The high-temperature compressive strengths with 30% iron ore carbonized at 700, 800, 900, and 1000°C were higher than 2000 N, which met BF requirements. The liquid phase content in the slag, which included impurities (Fe3O4, FeO, Al2O3, and SiO2) in the coal and iron ore, changed with the carbonization temperature. The liquid phase content of the slag was calculated by the FactSage 7.2 software and the results is shown in Fig. 5. It can be seen that the liquid phase content of the slag increased with increasing carbonization temperature and decreasing Fe3O4 content. Moreover, the Fe3O4 content decreased with increasing carbonization temperature which has been proved by the previous study.27) This decrease led to the existence of a large amount of the liquid phase in the slag, thus decreasing the high-temperature compressive strength of the iron coke. In addition, the slag content in the iron coke increased with increasing iron ore content. Therefore, with 30 and 40% iron ore, the high-temperature compressive strength increased initially and then decreased with increasing carbonization temperature.

Variation in the high-temperature compressive strengths of iron coke with varying carbonization temperatures, using (A) 20, (B) 30, and (C) 40% iron ore. (Online version in color.)

Liquid slag content with varying carbonization temperatures and Fe3O4 content, using (A) 40% iron ore and Fe3O4 content of 20% in the slag and (B) 40% iron ore and a carbonization temperature of 900°C. (Online version in color.)
The variations in the high-temperature compressive strength and mass loss with iron ore content are shown in Fig. 6. At a carbonization temperature of 900°C, the mass loss increased from 27.59 to 30.23%, while the high-temperature compressive strength decreased from more than 4200 to 1589 N, with an increase in the iron ore content from 20 to 40%. Thus, there was a negative correlation between the high-temperature compressive strength and mass loss at a carbonization temperature of 900°C. With an increase in the iron ore content, the liquid phase in the slag, iron oxide reduction, and CO2 gasification would increase, causing a decrease in the high-temperature compressive strength.

Variation in the high-temperature compressive strength and mass loss with the iron ore content. (Online version in color.)
The micromorphologies of iron coke with 20 and 30% iron ore carbonized at varying temperatures are shown in Figs. 7 and 8, respectively. Owing to the combined effects of coal coking, pitch volatilization, iron oxide reduction, and CO2 gasification, the porosity of the iron coke changed significantly. The ratio of the pore area (Ap) to the cross-sectional area of the image (Ao) varied with the carbonization temperature (Figs. 7 and 8, and drawn in Fig. 9). It is apparent that the porosity of the iron coke increased gradually with increasing carbonization temperature, owing to coal coking, the reduction reaction of iron oxides, and CO2 gasification strengthening with increasing carbonization temperature. It is known that the strength of coke originates from the pore wall structures.17,28,29,30) In this study, the pore walls mainly included slag, metallic iron, and the coke matrix. The addition of 20% iron ore to the briquette had no real effect on the pore walls and structure. Therefore, the high-temperature compressive strength increased with an increase in the carbonization temperature. The iron coke with 30% iron ore showed no significant difference in pore sizes at carbonization temperatures of 700 and 800°C. Numerous non-closed pores were formed as the carbonization temperature was increased from 800 to 1000°C, decreasing the high-temperature compressive strength at that temperature. With increasing iron ore and carbonization temperature, a large amount of slag (with a low melting point) and metallic iron was embedded in the coke matrix, decreasing the high-temperature compressive strength.

Micromorphology of iron coke with 20% iron ore carbonized at varying temperatures. Carbonization was conducted at (A) 700, (B) 800, (C) 900, and (D) 1000°C. (Online version in color.)

Micromorphology of iron coke with 30% iron ore carbonized at varying temperatures. Carbonization was conducted at (A) 700, (B) 800, (C) 900, and (D) 1000°C. (Online version in color.)

The micromorphologies of iron coke with varying contents of iron ore carbonized at 900°C are shown in Fig. 10, and the corresponding ratio of Ap to Ao is drawn in Fig. 11. Figures 10 and 11 show that the size and number of pores decrease while the number of metallic iron particles increases with increasing iron ore content. The embedding of numerous metallic iron particles in the matrix leads to easy fracture of the pore wall at high temperatures, thus decreasing the high-temperature compressive strength of the iron coke.

Micromorphology of iron coke carbonized at 900°C, using (A) 20, (B) 30, and (C) 40% iron ore. (Online version in color.)

Ratio of the pore cross-sectional area the changed with the iron ore content (obtained from Fig. 10).
There are two main processes involved in the coking process: coal particle bonding and coke strength generation. The bonding of coal particles is achieved by filling the space between the particles by dilatation, followed by shrinkage of the coal. Insufficient bonding between the particles lowers the coke strength. The addition of iron ore affects the swelling behavior and shrinkage behavior of the briquettes during coking, especially in the case of high iron ore additions, and further influences the strength.11,26) Coke strength generation is determined by the porosity and inherent strength.31) In this study, the strength of the iron coke was also determined by the bonding of the coal particles, porosity, and inherent strength of the pore wall. Coal coking, iron oxide reduction, pitch volatilization, and CO2 gasification significantly changed the micromorphology of the iron coke. Therefore, the high-temperature compressive strength was affected by these factors. Compared with coal process, the major differences in the carbonization of iron coke are iron oxide reduction, pitch volatilization, and CO2 gasification. The influence of pitch on the high-temperature compressive strength of iron coke can be overlooked because of the small amounts of asphalt added and volatilization at low temperatures. In iron ore reduction, most of the iron ore is reduced by C and CO, and a part of the iron ore is reduced by other reductants, such as H2. Therefore, the primary reduction reaction was a carbon-based reduction. The main reactions are listed in Table 4. CO2 gasification (above 900°C, CO2 + C = 2CO) involves the use of CO2 generated from the tail gas obtained by iron oxide reduction; as the reaction agent consumes the carbon in iron coke, its strength decreases. Therefore, the high-temperature compressive strength of iron coke with 30 and 40% iron ores decreases when carbonization at above 900°C. Besides, the carbon loss ratio was the main factor affecting the coke strength; there was a negative relationship between the compressive strength of coke and carbon loss ratio also proved above analysis.32,33) Compared with the coal process, the iron oxide reduction and further CO2 gasification consume carbon in the iron coke which is caused by the presence of iron ore, thus the iron ore content is the main factor directly affecting the high-temperature compressive strength of iron coke.
| Reaction | No. |
|---|---|
| 6Fe2O3 + C = 4Fe3O4 + CO2 | 1 |
| 2Fe3O4 + C = 6FeO+CO2 | 2 |
| 2FeO + C = 2Fe + CO2 | 3 |
| 3Fe2O3 + CO = 2Fe3O4 + CO2 | 4 |
| Fe3O4 + CO = 3FeO + CO2 | 5 |
| FeO + CO = Fe + CO2 | 6 |
| CO2 + C = 2CO | 7 |
During carbonization, the high-temperature compressive strength of the iron coke was mainly obtained from coal coking. Generally, the coke strength increases with the coking temperature.13,21,34) The carbonization mechanism of iron coke carbonized at 900°C is shown in Fig. 12. In this study, the high-temperature compressive strength of iron coke with 20% iron ore followed the above rule. Owing to the small amount of iron ore added, the influence on the high-temperature compressive strength was not reflected in the rule. As the iron ore content increased to 30 and 40%, the high-temperature compressive strength of the iron coke initially increased and then decreased with increasing carbonization temperature. The increase in the high-temperature compressive strength of iron coke at carbonization temperatures of 700 and 800°C is mainly due to the low reduction rate and metallization of iron ore at low temperatures, which have little influence on coal coking. With an increase in the carbonization temperature, the reduction rate of the iron ore increased, and a large amount of iron ore was reduced, increasing metallization. Therefore, there are three reasons for the decrease in the high-temperature compressive strength at the high carbonization temperature: 1) increase in carbon consumption; 2) embedding of a large amount of metallic iron in the carbon matrix; and 3) formation of a large amount of a low-melting-point slag phase (the impurity oxide from the iron ore and coal).
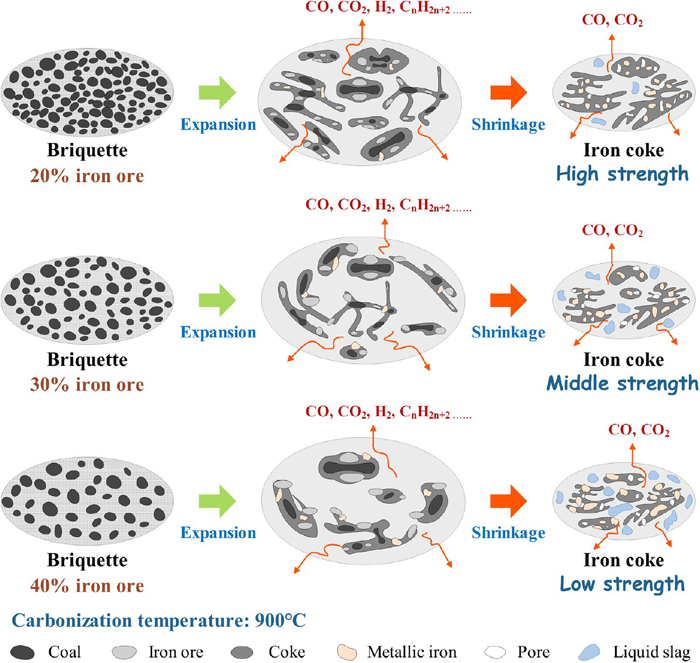
Diagram of carbonization mechanism of iron coke (carbonization at 900°C). (Online version in color.)
According to the compressive strength results, the high-temperature compressive strength of iron coke with 20% iron ore exceeded 2000 N at the carbonization temperatures of 800, 900, and 1000°C. The high-temperature compressive strength of iron coke with 30% iron ore was above 2000 N at carbonization temperatures of 700, 800, 900, and 1000°C. Meanwhile, the high-temperature compressive strength of iron coke with 40% iron ore was higher than 2000 N at a carbonization temperature of 800°C. Considering that the application of iron coke in BFs has certain energy-saving and emission-reduction effects, it is beneficial to use a high iron ore content in iron coke. However, the high-temperature compressive strength of iron coke with 40% iron ore was above 2000 N only at a carbonization temperature of 800°C. The temperature of the iron coke used in the BF is over 800°C. Thus, the use of iron coke with 40% iron ore is not recommended. In summary, the high-temperature compressive strength of iron coke with 30% iron ore was acceptable. The high-temperature compressive strength test results in this study are consistent with the cold strength results previously reported, which showed that the strength of iron coke is acceptable for BFs with an addition amount of 30%.11)
In this study, the in situ compressive strength of iron coke in high-temperature carbonization was investigated. The following conclusions were drawn.
(1) The mass loss increased with an increase in the iron ore content from 20 to 40%. The liquid phase content of the slag in the iron coke increased with the carbonization temperature, while the Fe3O4 content decreased.
(2) There was a negative correlation between the high-temperature compressive strength and mass loss at a carbonization temperature of 900°C. In addition, the mass loss increased from 27.59 to 30.23%, while the high-temperature compressive strength decreased from more than 4200 to 1589 N, with an increase in the iron ore content from 20 to 40%.
(3) The increase in the iron content in iron coke is beneficial for energy-saving and emission-reduction effects. The high-temperature compressive strength of iron coke with 30% iron ore and carbonization temperatures of 700, 800, 900, and 1000°C was acceptable.
(4) Coal coking, iron oxide reduction, pitch volatilization, and CO2 gasification significantly changed the micromorphologies and influenced the high-temperature compressive strength. There were three reasons for the decrease in the high-temperature compressive strength of iron coke: 1) increase in carbon consumption; 2) embedding of a large amount of metallic iron in the carbon matrix; 3) formation of a large amount of a low-melting-point slag phase.
This work was supported by the National Natural Science Foundation of China-Liaoning Joint Funds (Grant No. U1808212); National Natural Science Foundation of China (Grant No. 52104325); National Natural Science Foundation of China (Grant No. 52074080).
The authors report there are no competing interests to declare.