2023 Volume 72 Issue 1 Pages 1-10
2023 Volume 72 Issue 1 Pages 1-10
Vesicles (liposomes and niosomes) are bilayer membranous capsules composed of amphiphilic molecules having aqueous phase in their interior and can encapsulate drug ingredients to act as drug delivery systems, a bio-membrane model, and so on. Vesicles also find their applications in cosmetics and foods industries since they can not only entrap water-soluble substances in their core, but also solubilize oily substances in the bilayer membrane. Almost half a century has passed since the discovery of vesicles by Bangham, and research on their basic properties and applications has been gaining momentum once again. In this article, the preparation and properties of vesicles (liposomes, niosomes) with excellent dispersion stability, especially formed in mixtures of amphiphilic molecules, are reported. Furthermore, the preparation of nano-sized silica hollow particles using vesicles as a structure-directing agent and their application to anti-reflection film are also described.
A vesicle is a bilayer membrane capsule made of amphiphilic molecules. The use of vesicles as drug carriers in drug delivery systems (DDSs) is being actively investigated. Liposomes are phospholipids vesicles and can serve as artificial cell membrane models because of their similarity to cell membrane structures. Vesicles can retain water-soluble substances in their interior and can retain (i.e., solubilize) oil-soluble substances in bilayer membranes; therefore, they have been used in biological applications as well as in a wide range of other fields such as cosmetics, foods, and chemicals. A cryo-transmission electron microscopy (TEM) image of the vesicles formed by cationic surfactants is shown in Fig. 1. Studies on vesicles began with the discovery of bilayer membrane structures (liposomes) comprising phospholipids by Bangham et al. in 1964 1) . This was followed by a pioneering study by Kunitake et al. on vesicle formation using synthetic amphiphilic molecules in 1977 2) . Currently, vesicle formation by amphiphilic molecules with various molecular structures and the formation of vesicles with superior dispersion stability in mixed systems of amphiphilic molecules have been reported 3) , 4) , 5) , 6) , 7) , 8) , 9) , 10) , 11) . Liposomes are formed by phospholipids 3) , 4) , 5) , whereas vesicles formed by non-ionic surfactants are called niosomes 6) , 7) , 8) , and vesicle surfaces coated with silica are called cerasomes 11) . Recently, studies on exosomes 12) , which are extracellular vesicles enclosed in lipid bilayer membranes secreted by cells, have attracted considerable research attention.

Cryo-TEM image of vesicles.
Nearly half a century after the discovery of vesicles by Bangham 1) , studies on their basic properties and applications have gained momentum. This study first presents an overview of the conditions for vesicle formation and the factors that govern vesicle stability. Subsequently, preparation methods for vesicles with excellent dispersion stability in amphiphilic mixed molecular systems are introduced. Furthermore, the preparation of nanosized silica hollow particles using vesicles as structure directing agents has also been reported.
A useful and informative indicator for understanding the type and phase state of molecular aggregates formed by amphiphilic molecules is the critical packing parameter (CPP) 13) , 14) .
 |
where, a 0 is the effective cross-sectional area of the hydrophilic group, V denotes the volume of the hydrophobic group, and l c denotes the chain length of the hydrophobic group. CPP is a measure of the geometry of a single surfactant molecule, which determines the structure that the surfactant can assemble, that is, the shape and type of the molecular assembly. Surfactant molecules form spherical micelles, cylindrical micelles, vesicles, and planar membranes when the CPP is below 1/3, lies between 1/3 and 1/2, ranges from 1/2 to 1, and approaches 1, respectively. Notably, vesicles are usually in a metastable state. For example, double-chain cationic surfactants, such as didodecyldimethylammonium bromide (DDAB), have a molecular structure (CPP~1) that easily forms a planar bilayer membrane. Therefore, the vesicles are temporarily dispersed in water when an external force such as ultrasound is applied. However, over time, the vesicles grow, and eventually the flat lamellar phase separates in a state of thermodynamic equilibrium. Thus, CPP alone cannot explain the stability of vesicles.
Therefore, we considered the destabilizing factors of vesicles and flat lamellae with respect to the same bilayer membrane structure. First, in flat lamellae, the hydrophobic groups of the terminal molecules are thermodynamically unstable (i.e., have a high edge energy) because they are in contact with water. Therefore, infinite association was performed to minimize the number of terminal molecules. Two possible methods can be employed to resolve the edge instability of the bilayer membranes.
First, the bilayer membrane ends are “capped” by arranging the hydrophilic groups toward the outside (water side) so that the hydrophobic groups do not come into contact with water at the edges. However, even though the curvature of the bilayer membrane is zero, the cap portion has a large positive curvature, forcing capped molecules to pack forcibly. In other words, the cap portion must be composed of molecules with a CPP smaller than that of the bilayer membrane (body portion).
Another solution to prevent edge destabilization is to form a closed shell or vesicle with completely closed edges. The curvature of the flat lamellae is zero; however, vesicles have curvature. Therefore, the instability of the vesicles is related to the elastic curvature energy of the bilayer membrane. Safran et al. 15) expressed the curvature elastic energy per unit area of a bilayer membrane E using the following equation:
 |
Where κ is the curvature elastic modulus, c is the spontaneous curvature of the bilayer membrane, co is the outer membrane curvature, and ci is the inner membrane curvature. The curvature elastic modulus κ is related to the fluidity of the bilayer membrane, and the more fluid-like (softer) the bilayer membrane, the lower the curvature elastic modulus. As Fig. 2 shows, the outer monomolecular film of the bilayer membrane of vesicles has a positive curvature, and the inner monolayer has a negative curvature. Therefore, deviation from the spontaneous curvature of each membrane is a destabilizing factor. Therefore, to form stable vesicles (i.e., decrease the curvature elastic energy), interfaces with opposite curvatures of positive and negative signs must be simultaneously achieved.

Relationship between outer and inner monolayer curvatures in vesicle bilayer membranes.
As previously indicated, to reduce the curvature elastic energy, which is a destabilizing factor for vesicles, the outer and inner monolayers of the bilayer membrane should preferentially contain molecules with small and large CPPs to induce positive and negative curvatures, respectively. A typical example of a system that can achieve this is an anion/cationic surfactant mixed system. Kaler et al. 16) , 17) , 18) reported that a highly stable vesicle spontaneously forms by simply mixing anionic and cationic surfactants in water. In an anion/cation mixed system, a pseudo-double-tailed complex suitable for bilayer membrane formation is formed by electrostatic attraction between hydrophilic groups. Therefore, compositions other than equimolar ratios can be regarded as mixed systems of excess single-tailed components of a small CPP and pseudo-double-chain complex of a large CPP. The excess single component in the bilayer membrane or the pseudo-double-chain complex preferentially aligns with the outer or inner monolayer, respectively, which reduces the curvature elastic energy and stabilizes the vesicle shape. Thus, anion/cationic surfactant mixed systems have attracted research attention because they form vesicles with high dispersion stability. However, their disadvantage is that the charge of the hydrophilic group is completely shielded near the equimolar ratio, resulting in gradual precipitation.
3.2 Stable vesicle formation in glycerol-modified cationic surfactant/anionic surfactant mixed systemsTo suppress precipitation near the equimolar ratio, we examined the phase behavior of a mixed system of cationic surfactant monoglycerylcetyldimethylammonium chloride (MGCA, Fig. 3) in which the glycerin moiety was introduced into the hydrophilic group and anionic surfactant sodium octyl sulfate (SOS). Further, we attempted to expand the compositions to form vesicles with better dispersion stability 19) .

Molecular structure of glycerol-modified cationic surfactant MGCA.
Figure 4 shows the phase diagram (20°C) of the MGCA/SOS/water ternary system. A cetyltrimethylammonium chloride (CTAC) /SOS/water ternary phase diagram using a typical cationic surfactant, CTAC, instead of MGCA, is also presented for comparison. Results showed that precipitation occurred over a relatively wide range close to the equimolar ratio in the CTAC/SOS mixed system, whereas no precipitation occurred in the MGCA/SOS mixed system, except in the dilute region (with a total surfactant concentration of 30 mM or less). It was also observed that vesicles were formed over a wider area in the MGCA/SOS mixed system than in the CTAC/SOS mixed system. Figure 5 shows a cryo-TEM image of the MGCA/SOS mixed system (MGCA/SOS=1/1, total concentration: 50 mM). Results showed that despite equimolar mixing, unilamellar vesicles 30-70 nm in diameter were formed. Figure 5 also shows the change in the average particle size over time in equimolar compositions of the MGCA/SOS and CTAC/SOS mixed systems, as determined by dynamic light scattering (total concentration: 50 mM). In the CTAC/SOS mixed system, the particle size increased quickly after preparation, and precipitation occurred after one month. In contrast, the average particle size in the MGCA/SOS mixed system increased slowly until three months after preparation, after which it remained constant. It was found that vesicles formed in a wide range of regions in the MGCA/SOS mixed system, and the vesicle dispersion stability was high.

Phase diagrams of (a) MGCA/SOS/water ternary system and (b) CTAC/SOS/water ternary system at 20°C (M: spherical micelle, R: rod micelle, V: vesicle, L: lamellar liquid crystal, P: precipitation).
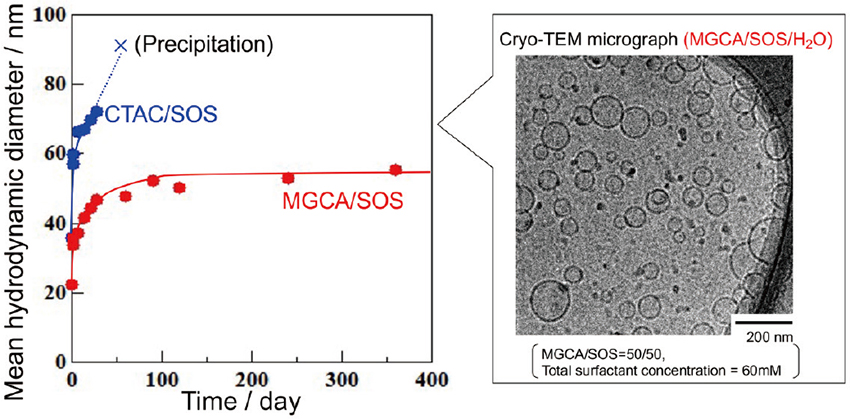
Cryo-TEM image and change in average particle size over time of the vesicles formed by MGCA/SOS/water ternary system (total surfactant concentration: 50 mM; MGCA/SOS=1/1).
In addition, fluorescence polarization measurements were performed to investigate the vesicle dispersion stabilization factors in the MGCA/SOS mixed systems. Fluorescence polarization is an indicator of the fluidity of a bilayer membrane, where the lower the fluorescence polarization, the higher the fluidity of the bilayer membrane. The results showed that the fluorescence polarization decreased sharply at approximately 25°C in the CTAC/SOS mixed system. This indicated that below 25°C, the bilayer membrane was in a gel-like state with dense packing and low fluidity, whereas above 25°C, it was in a liquid crystal state with sparse packing and high fluidity. However, fluorescence polarization decreased slowly with increasing temperature in the MGCA/SOS mixed system. The fluorescence polarization at 20°C was lower for the MGCA/SOS mixed system than for the CTAC/SOS mixed system. Therefore, one factor contributing to the formation of vesicles with high dispersion stability in the MGCA/SOS mixed system may be the formation of a bilayer membrane with high fluidity (i.e., low curvature elastic energy), even at low temperatures. Even if the charge of the surfactant hydrophilic group is shielded in the equimolar ratio, the strong hydration repulsion caused by the hydration of the glycerin moiety of MGCA is considered to suppress vesicle aggregation.
Niosomes are vesicles composed of non-ionic surfactants. Similar to liposomes formed from phospholipids, niosomes have attracted attention as carriers for DDSs. Niosomes are expected to be promising alternatives to phospholipids because they are inexpensive, stable, and suitable for long-term storage 7) , 8) , 20) , 21) , 22) , 23) , 24) . In addition, previous reports on niosomes have shown that, when applied as a topical transdermal formulation, they reduce side effects, continue to release the encapsulated substance, and increase its permeability into the skin 22) . Non-ionic surfactants can freely select the molecular structure of the hydrophilic group and adjust the hydrophilic/hydrophobic balance of the molecule, facilitating the control of the physical properties of the bilayer membrane of the formed niosome. Therefore, optimal niosome formulations are being considered for various applications.
4.2 Preparation of niosomes using supercritical carbon dioxide reverse phase evaporation methodAs with liposomes, several methods of preparing niosomes utilize organic solvents such as chloroform, and concerns have been raised about the biological and environmental effects of these residues. In contrast, we focused on supercritical carbon dioxide (sc-CO2) as an alternative solvent and reported the preparation of liposomes 25) , 26) , 27) and niosomes 28) , 29) by sc-CO2 reverse phase evaporation (sc-CO2-RPE) method using sc-CO2 as a solvent (Fig. 6). In the preparation of niosomes using this method, polyoxyethylene non-ionic surfactants were initially studied; however, they were normally considered unsuitable for use in the food and pharmaceutical fields. In this study, we present the results of niosome preparation using the scCO2-RPE method using polyglycerol fatty acid esters (PGFEs), which were synthesized from plant-derived raw materials and used as food additives 30) .

Vesicle formation process by sc-CO2-RPE methods.
PGFEs with various hydrophilicity/lipophilicity balance (HLB) values, as shown in Table 1 were used. The PGFEs were dissolved in ethanol, an auxiliary solvent, and introduced into a pressure-resistant cell. The solvent was warmed to 60°C and pressurized to 130 bar by filling it with CO2 to create a supercritical state. A predetermined amount of water was then added, and CO2 was expelled to prepare niosomes (see Fig. 6).
The formation of niosomes was confirmed by cryo-TEM observation of decaglycerin distearate (DG2S) with two added molar numbers of stearic acid and an HLB of 9.5, and in decaglycerin diisostearate (DG2IS) with an HLB of 10. Comparison of the retention efficiencies of water-soluble glucose showed that DG2IS had higher values. Furthermore, fluorescence anisotropy measurements using diphenylhexatriene, an oil-soluble fluorescent probe, showed that the DG2IS system had lower fluorescence anisotropy values. Fluorescence anisotropy is related to membrane fluidity; low values of fluorescence anisotropy indicate high membrane fluidity. Therefore, DG2IS is considered to have higher membrane fluidity and can exist stably even in a state with curvature, which improves the long-term stability and retention efficiency of niosomes.
To determine the effects of the different HLB values of non-ionic surfactants on niosome formation, we examined whether niosomes could be prepared by mixing DG1S and DG3S, which have high and low HLB values, respectively, and cannot form vesicles alone. TEM observations of niosomes prepared at various mixing ratios revealed that niosomes with unilamellar structures were formed by adjusting the composition such that the HLB was 9-10 (Fig. 7). Accordingly, we determined that niosomes can be prepared by mixing surfactants that do not form niosomes in a single system and by adjusting the HLB to an appropriate value. In addition, the dispersion stabilities of niosomes prepared in surfactant alone (DG2S) and in mixed systems (DG1S/ DG3S) were evaluated by visual observation and changes in particle size over time. Phase separation occurred within 4 d in the single system; however, it remained stable after 2 weeks in the mixed system. Figure 8 shows the time-course change in niosome size prepared with a mixture of DG1S/DG3S and a single DG2S system. Niosomes prepared with DG1S/DG3S mixtures maintained their size for a longer period (one week) than those prepared with DG2S alone (1 d). This was thought to be because the mixed system of surfactants suppressed the rigid packing of the outer and inner membranes that made up the bilayer membrane.

Cryo-TEM image of niosomes formed by a PGFE mixed system (DG1S and DG3S).

Time course change in niosome size prepared by a mixture of DG1S/ DG3S and DG2S alone.
From the aforementioned results, we concluded that the dispersion stability of vesicles (niosomes) formed by non-ionic surfactants could be improved by mixing surfactants with different critical packing parameters.
Considerable research has been conducted on the preparation of nanostructured materials (e.g., metal oxides, metals, and other inorganic materials) using various molecular assemblies formed using surfactants as templates or structure-directing agents. We previously reported the preparation of nanoporous titania with crystalline wall films 31) , 32) , nanoporous silica with various pore structures 33) , and noble metal/titania-core/shell nanoparticles 34) , 35) , 36) . In contrast, formation of nanosized hollow particles can be expected if sol/gel reactions of the precursor molecules can be selectively promoted on the surfaces of vesicles.
Hollow silica particles are expected to be applied to anti-reflective films and DDS carriers because of their low refractive indices and ability to encapsulate materials. In general, hollow silica particles are prepared by a hard template method using polystyrene latex particles or similar templates 37) or by a soft template method using molecular aggregates or emulsions formed by surfactants as templates 38) . However, obtaining highly dispersible hollow particles with sizes of 100 nm or less is difficult.
Therefore, we studied the preparation of hollow silica particles using vesicles formed in cationic surfactant mixed systems as structure-directing agents 39) , 40) . The structure-directing agent is a vesicle with excellent dispersion stability (average particle size of approximately 100 nm) formed by a mixed system of DDAB and dodecyltrimethylammonium bromide (DTAB), which have double- and single-chain structures, respectively. We previously found that hydrolysis and polycondensation reactions of tetraethyl orthosilicate (TEOS), a silica precursor, in the presence of the aforementioned vesicles in an aqueous ammonia solution with an approximate pH of 11.5 could produce nano-sized silica hollow particles with a particle diameter of 30-70 nm. However, the agglomeration between particles is severe, making it unsuitable for practical applications. In this section, we introduce a method we recently developed for preparing highly dispersible silica hollow particles of approximately 50 nm in diameter based on a step-wise change in pH 41) .
The vesicles formed in the DDAB/DTAB mixed system were used as soft templates. TEOS was added to initiate the sol/gel reaction at various pH levels, and the pH was changed during the reaction. In particular, we studied a method to change the pH in the initial stage of the ammonia basic reaction, which produces fine hollow particles (but also causes agglomeration), to a pH in the neutral region, where the dissolution process of silica does not occur during the reaction, and then to complete the reaction.
Results showed that when TEOS was added at pH 11.5, stirred for 2 h, and then pH was changed to 7 or 8 with continued stirring for 12 h, a solution with a bluish-white color and excellent dispersion stability was obtained (Fig. 9). In addition, TEM observations and dynamic light scattering measurements of this sample confirmed that independent hollow silica particles of 30-50 nm in diameter were obtained (Fig. 9). The resulting hollow silica particles were also found to be stably dispersible in water for at least one year. Fourier transform infrared and thermogravimetric measurements clarified that a sufficient hydration layer was formed on the silica particle surface after hydrothermal treatment when the pH was changed to the neutral region during the reaction. This suggests that the hydration layer is a critical factor in improving the dispersion stability. In addition, although the size of the vesicles used as structure-directing agents in this study was approximately 100 nm, the size of the resulting silica hollow particles was smaller, approximately 50 nm. This was because the hydrolysis products of TEOS were adsorbed onto the vesicle surface, which destabilized the vesicles. These were then transformed into smaller molecular aggregates through the application of a shearing force by stirring in this state.

TEM images and appearance of highly dispersive silica hollow particles obtained by step-wise change in pH with vesicles formed in a cationic surfactant mixture as the structure-directing agent.
We examined the application of the highly dispersible hollow silica particles in anti-reflection films. Because of the low refractive index of silica among inorganic substances, films coated with hollow particles are expected to develop into antireflection films with superior mechanical strength compared to those coated with organic substances. In our study, thin films were prepared by coating hollow particle dispersions on glass substrates by a “layer-by-layer” method using polyelectrolytes, and the change in transmittance in the visible light region was evaluated. The results showed that the transmittance increased from 90% to 93.5%, indicating that the resulting silica hollow particle-coated film showed anti-reflection properties. In addition, the size of the silica hollow particles was approximately 50 nm, which enables high permeability and retention in cells (i.e., the enhanced permeability and retention effect). Therefore, it is expected that by encapsulating radioactive therapeutic agents inside hollow particles, these particles can be used as drug carriers for boron neutron capture therapy.
The properties and functions of vesicles (liposomes and niosomes) obtained by “nanoarchitectonics” of amphiphilic molecules have been described in this review article, focusing on our recent research. The formation of stable vesicles using various amphiphilic molecules such as phospholipids and non-ionic surfactants has recently become possible, and their application in cosmetics, foods, and resin additives has been actively pursued. These vesicles can also be used as templates and structure-directing agents for the preparation of inorganic nanomaterials, such as hollow particles or nanotubes. We have also recently reported vesicles with novel functionalities, including stimulus-responsive vesicles that can switch the aggregation state by light irradiation 42) and vesicles that can be polymerized and microencapsulated 43) . In addition, the mechanism of vesicle formation and its dynamics are related to the mechanism of exosome formation from cells, and more detailed studies on this topic are necessary. We look forward to further progress in both, the basic and applied aspects of vesicle-related research, with the aid of advanced evaluation techniques.
I express our deepest gratitude to Professor Masahiko Abe, Dr. Kenichi Sakai, Dr. Koji Tsuchiya, Dr. Shunsuke Yamaguchi, Dr. Takeshi Endo, Dr. Taku Ogura, Dr. Masaaki Akamatsu and many other coworkers as well as many students for their support in conducting our research on vesicles.