2015 Volume 40 Issue 3 Pages 124-129
2015 Volume 40 Issue 3 Pages 124-129
Sensitivities to ipconazole of 50 isolates of the Fusarium (Gibberella) fujikuroi species complex (Ff complex) obtained from rice in Japan before 1994 (group A: before the launch of ipconazole) and 65 isolates after 1994 (group B) were investigated. Most isolates were identified as Fusarium fujikuroi, while 5 isolates in group A and 4 isolates in group B were identified as Fusarium proliferatum with species diagnostic PCR-RFLPs. The minimum inhibitory concentration (MIC) range, profiles in sensitivity to ipconazole and gibberellin production were similar in both groups. Gibberellin production was detected in the F. fujikuroi isolates with higher sensitivity to ipconazole but not in the isolates with lower sensitivity and F. proliferatum. High efficacy of ipconazole against rice “Bakanae” disease was confirmed by using the gibberellin-producing F. fujikuroi isolates in group B. No shift in sensitivity to ipconazole was verified and this may contribute to the stable efficacy against rice “Bakanae” disease.
Ipconazole, (1RS,2SR,5RS; 1RS,2SR,5SR)-2-(4-Chlorobenzyl)-5-isopropyl-1-(1H-1,2,4-triazol-1-ylmethyl) cyclopentanol, was synthesized and its biological activity was found by Kureha Corporation in 1986.1) It functions as a demethylation inhibitor (DMI) of the fungal ergosterol biosynthesis pathway. Ipconazole was firstly introduced in 1993 in Japan as a seed disinfectant for rice (trade name: Techlead®) and it had been globally registered and being used as a seed disinfectant for cereals, corn, cotton and other kinds of corps in more than 20 countries by 2014. The characteristic of ipconazole as a seed disinfectant has been described previously.2)
Recent chemical fungicides have favorable safety and target selectivity profiles. However fungicides with a single mode of action face resistance issues. Therefore, the data concerning sensitivity of pathogens to a selective fungicide provide a good basis for preparing appropriate strategies to control the resistant strains more effectively. Directional selection, a shift toward a less sensitive direction by multiple mechanisms of polygenic dominance, is observed in DMI resistance and uncontrollable less sensitive pathogens have gradually increased.3)
“Bakanae” disease is one of the most important seed-born diseases in rice caused by Fusarium fujikuroi Nirenberg (teleomorph: Gibberella fujikuroi [Sawada], Ito in Ito & Kimura). Formerly, under broad species concept based on morphology, F. fujikuroi had been treated as F. moniliforme. However, this inclusive taxon was abolished4) and F. fujikuroi is currently a member of the Fusarium (Gibberella) fujikuroi species complex (Ff complex) based on a phylogenetic species concept. Thus far, 11 mating populations (MPs), A through K, have been found in the Ff complex and F. fujikuroi corresponds to MP-C.
The sensitivity of the former F. moniliforme isolates to ipconazole was previously reported.5) The minimum inhibitory concentration (MIC) of the isolates to ipconazole ranged from 0.10 to 6.25 mg/L with the main peak at 0.39 mg/L and the shoulder at 3.13 mg/L. Pathogenicity to rice and gibberellin production were detected in the isolates with MIC values lower than or equal to 0.78 mg/L, while both were at undetectable level in the isolates with higher MICs. Approximately 60 µg/g seeds of ipconazole was deposited in the rice seeds after the seed treatment with a practical dosage (at germination stage).3) Based on observation, it was suggested that the narrow range of MIC and adequate margin between the highest MIC to the pathogenic isolates and deposit of ipconazole in seeds may prevent development of resistance and contribute to stable efficacy against “Bakanae” disease.5)
In this paper sensitivity to ipconazole, species identification and gibberellin production in Ff complex from rice in Japan before (group A) and after (group B) the launching ipconazole were compared. In addition, efficacy of ipconazole against rice “Bakanae” disease was evaluated by using gibberellin-producing F. fujikuroi isolates in group B.
Fusarium isolates used in this study are shown in Table 1. Production of microconidial chains in aerial mycelia, one of the morphological characteristics of former F. moniliforme, was confirmed by microscopy. Fifty isolates obtained before 1994 (group A: before the practical use of ipconazole) were randomly chosen from 211 F. moniliforme isolates which were used in previous ipconazole sensitivity study.5)
| Code of isolates | Number of isolates | Prefecture | Isolated part of rice plant | Year of isolation |
|---|---|---|---|---|
| Group A (Isolates obtained before 1994)a) | ||||
| AKT 1 | 1 | Akita | not specified | Before 1991 |
| AOM 93-3, 4 | 2 | Aomori | elongated stem | 1993 |
| EHM 92-1, 2 | 2 | Ehime | elongated stem | 1992 |
| GIF 89-2 | 1 | Gifu | elongated stem | 1989 |
| HKD 1 | 1 | Hokkaido | not specified | Before 1991 |
| HRS 93-3, 5 | 2 | Hiroshima | elongated stem | 1993 |
| IBR 89-1 | 1 | Ibaraki | not specified | Before 1989 |
| IWT 88-2, 92-8 | 2 | Iwate | elongated stem | 1988 |
| KGS 93-10, 16 | 2 | Kagoshima | not specified | 1993 |
| KGW 91-4 | 1 | Kagawa | seed | 1991 |
| KGW 1 | 1 | Kagawa | not specified | Before 1991 |
| KMM 92-7, 8 | 2 | Kumamoto | elongated stem | 1992 |
| MIE 92-1, 2, 4 | 3 | Mie | elongated stem | 1992 |
| MYG 92-1, 10, 12 | 3 | Miyagi | elongated stem | 1992 |
| MYG 93-1 | 1 | Miyagi | elongated stem | 1993 |
| NGN 92-1, 5, 6, 13, 15 | 5 | Nagano | elongated stem | 1992 |
| NGT 89-3 | 1 | Niigata | seed | 1989 |
| OKY 91-1, 6 | 2 | Okayama | elongated stem | 1991 |
| OKY 93-4, 9, 11, 12 | 4 | Okayama | elongated stem | 1993 |
| SMN 86-2 | 1 | Shimane | elongated stem | 1986 |
| TCG 91-2 | 1 | Tochigi | elongated stem | 1991 |
| TCG 92-2 | 1 | Tochigi | elongated stem | 1992 |
| TTR 88-4, 12, 14, 17 | 4 | Tottori | elongated stem | 1988 |
| TYM 91-4 to 6 | 3 | Toyama | elongated stem | 1991 |
| YMG 92-2, 3, 15 | 3 | Yamagata | elongated stem | 1992 |
| Total | 50 | |||
| Group B (Isolates obtained after 1994)b) | ||||
| HKD 07-1 to 3 | 3 | Hokkaido | seed | 2007 |
| HKD 08-1 to 3 | 3 | Hokkaido | elongated stem | 2008 |
| HRS 09-3 | 1 | Hiroshima | seed | 2009 |
| KGW11-1 to 32 | 32 | Kagawa | elongated stem | 2011 |
| MIE 94-1 to 3 | 3 | Mie | elongated stem | 1994 |
| MYG 09-1, 2 | 2 | Miyagi | seed | 2009 |
| SIG 06-1 to 8 | 8 | Siga | elongated stem | 2006 |
| SIG 09-1, 3, 5, 7 | 4 | Siga | elongated stem | 2009 |
| SIG 09-2, 4, 6, 8, 9 | 5 | Siga | seed | 2009 |
| YMG 09-1 to 4 | 4 | Yamagata | seed | 2009 |
| Total | 65 |
a) Supplied from 22 prefectural research institutes for agriculture related industries. b) Supplied from Gifu University and Kagawa Prefectural Agriculture Experiment Station.
Sixty-five isolates obtained after 1994 (group B), were supplied from Gifu University and Kagawa Prefectural Agricultural Experiment Station. Among these 65 isolates, 62 were obtained between 2005 and 2011.
2. Extraction of genomic DNA of the isolatesThe isolates were shaken in potato dextrose broth medium (Difco™, Becton, Dickinson and Co., MD, USA) for 1 week at 25°C. The cultures were centrifuged at 5000×g (himac CR20 GII, Hitachi Koki, Ibaraki, Japan) and the medium supernatant was removed to obtain mycelial pellets. Liquefied nitrogen was poured over the pellets and pounded in a mortar. Approximately 30–50 mg of mashed mycelia was transferred to a microtube and genomic DNA was extracted and purified by using DNA extraction kit (DNeasy™ Plant Mini Kit, Quiagen, MD, USA).
3. PCR-RFLPs for species identification in the Ff complexCombinational PCR-RFLPs of the translation elongation factor 1α (TEF) gene and the histone H3 (HIS) gene were performed according to Suga et al. (2014).6) The TEF-PCR product was subjected to Dde I digestion and HIS-PCR product was subjected to double-digestion with Dde I and Hha I. The following isolates were used as standards in every agarose gel electrophoresis: F. verticillioides MAFF240087 (TEF PCR-RFLP pattern: I/HIS PCR-RFLP pattern: I), F. fujikuroi MAFF2838531(III/III), F. proliferatum MAFF410715(IV/III), F. sacchari MAFF239074(II/II).
4. Determination of MICsEach mycelial mass was transferred from original stock culture to a PDA plate and precultured for 7 days at 25°C. Ipconazole 6% wettable powder (Kumiai Chemical Industry Co., Ltd.) was dispersed in sterilized water and added to PDA medium at 10% of the final volume to obtain the final concentrations; 0.05, 0.10, 0.20, 0.39, 0.78, 1.56, 3.13, 6.25, 12.5, 25, 50 and 100 mg/L. From the colonies on precultured plate, 4 mm-diameter of mycelial disks were cut out and placed on PDA medium containing prescribed concentration of ipconazole and the plates were incubated for 5 days at 25°C. Growth of each isolate was observed and MIC was determined. The test has 3 replicates.
5. Determination of gibberellin productionThe mycelial mass was transferred from the stock culture to PDA plates and preincubated for 7 days at 28°C. Then mycelial disks 4 mm in diameter were cut out and transferred to 100 mL of Richard’s medium (KNO3 10 g, KH2PO4 5 g, MgSO4·7H2O 2.5 g, FeCl2 0.02 g, sucrose 30 g in distilled water 1000 mL, pH 5.4). The inoculated medium was cultured for 5 days at 28°C with a reciprocal shaker at 140 rpm. Bioassay of the gibberellins of these liquid culture filtrates was performed by using Nishijima’s method on a dwarf rice cultivar, Tanginbozu.7) Gibberellin production was determined by significant elongation of the second leaf sheath as compared with the blank reference (F-test).
6. Seed treatment with ipconazole and evaluation of its efficacy against rice “Bakanae” diseaseMycelial masses from the original isolates were transferred to and spread on PDA medium and incubated for 10 days at 25°C under fluorescent lighting. The microconidia formed on the media were collected and suspended in deionized water. The concentrations of the conidial suspensions were 106 to 107 spores/mL.
The rice seeds (cv. Tanginbozu, a dwarf cultivar) were dipped in and inoculated with the conidial suspension for 1 hr under vacuum. The volumetric ratio of the rice seeds to the conidial suspension was 1 : 1. The inoculated seeds were spread on a paper towel and dried for 24 hr at room temperature. Then the seeds were then used for the seed treatment efficacy test.
Wettable powder containing 6% ipconazole and flowable formulation containing 6% ipconazole and 5% copper hydroxide were used. Both fungicidal formulations were produced by Kumiai Chemical Industry Co., Ltd. Fifteen grams of the inoculated seeds was dipped in 30 mL of 200-fold dilution of each fungicide formulation for 24 hr at 15°C.
The fungicide-treated seeds were soaked in water at 15°C for 4 days (water-soaking) and the seeds were incubated at 30°C for 3 days for germination. The volumetric ratio of the seeds to water was 1 : 1 for water soaking and germination. The seeds were divided into three equal portions and sown on the granulated nursery soil in 10×15 cm plastic boxes and incubated at 30°C for 3 days for shooting. The seedlings were kept in the greenhouse until the third-leaf stage.
Efficacy against “Bakanae” disease was evaluated by counting the number of elongated and damping-off seedlings. The rates of diseased seedlings were compared with those of the untreated plots and protective values were calculated.
The species of group A and group B isolates were identified with species-diagnostic PCR-RFLPs for Ff complex. Based on the PCR patterns described previously,6) all but 1 isolate were identified as F. fujikuroi (TEF PCR-RFLP pattern: III/HIS PCR-RFLP pattern: III) or F. proliferatum (IV/III) (Data table not shown).
In 50 isolates of group A, which were obtained as F. moniliforme before 1994,5) 45 and 4 isolates were identified as F. fujikuroi and F. proliferatum (OKY 91-6, KGW 1, GIF 89-2, EHM 92-2), respectively. The other 1 isolate (NGN 92-1) was unidentified by this PCR-RFLP.
Among 65 isolates of group B, which were obtained after 1994, 61 and 4 isolates were identified as F. fujikuroi and F. proliferatum (HRS 09-3, YMG 09-4, SIG 09-8, SIG 09-9) respectively.
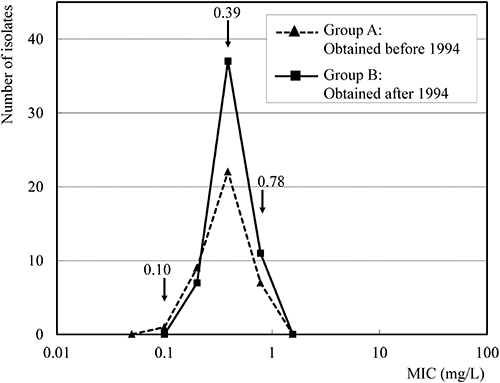
2. Comparison of sensitivity to ipconazole and gibberellin production of the Ff complex isolatesIn group A, the MIC values of the F. fujikuroi isolates to ipconazole ranged from 0.10 to 3.13 mg/L with a main peak at 0.39 mg/L. Both gibberellin producers and non-producers existed in the isolates with an MIC of 0.78 mg/L. However, all 39 isolates with MICs from 0.10 to 0.39 mg/L (high sensitivity) were gibberellin producers, while 6 isolates with MICs from 0.78 to 3.13 mg/L (low sensitivity) were gibberellin non-producers. The MIC values of the 5 F. proliferatum isolates ranged from 3.13 to 6.25 mg/L and none of them produced gibberellin (Fig. 1).

In group B, the MIC values of the F. fujikuroi isolates to ipconazole ranged from 0.20 to 6.25 mg/L with the main peak at 0.39 mg/L and minor peak at 3.13 mg/L. All 55 isolates with MICs from 0.20 to 0.39 mg/L (high sensitivity) were gibberellin producers, while 6 isolates with MICs from 1.56 to 6.25 mg/L (low sensitivity) were gibberellin non-producers. The MIC values of the 4 F. proliferatum isolates ranged from 1.56 to 6.25 mg/L and gibberellin was not detected in any of these isolates (Fig. 1).
Concerning the gibberellin production of F. fujikuroi, the MIC values of gibberellin producers in group B ranged from 0.2 to 0.78 mg/L with a single peak at 0.39 mg/L. This MIC range and profile were almost the same as that of gibberellin producers of F. fujikuroi in group A (Fig. 2). The MIC values of gibberellin non-producers in group B ranged from 0.78 to 6.25 mg/L. This MIC range was within that of gibberellin non-producers of F. fujikuroi in group A (Fig.1). The sensitivity and gibberellin production profiles were similar in both groups
The efficacy of ipconazole against “Bakanae” disease by seed treatment was evaluated using F. fujikuroi isolates randomly chosen from group B. The disease incidence of gibberellin producers (MICs: 0.20 to 0.78) were ranged from 11.5 to 100% without seed treatment and that of non-producers (MICs: 1.56 to 6.25 mg/mL) was 0%. The 200-fold 24-hr dipping treatment with ipconazole wettable powder and ipconazole-copper hydroxide flowable showed high efficacy (protective values were larger than 99.7) against “Bakanae” disease caused by all gibberellin producers (Table 2).
| Code of isolates | MIC for ipconazole (mg/L) | Gibberellin productivitya) | Spore suspensions as inoculab) (spores/mL) | Untreated (Dipping in water for 24 hr) | Ipconazole + copper hydroxide (5% + 4.5%) flowable 200-hold 24 hr dipping | Ipconazole (6%) wettable powder 200-hold 24 hr dipping | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number of seedlings | Incidencec) (%) | Total number of seedlings | Incidencec) (%) | Protective valued) | Total number of seedlings | Incidencec) (%) | Protective valued) | ||||
| HKD 08-2 | 0.20 | + | 3.2×106 | 387 | 99.0 | 350 | 0.0 | 100 | 346 | 0.0 | 100 |
| MYG 09-2 | 0.20 | + | 7.0×106 | 354 | 98.3 | 381 | 0.0 | 100 | 373 | 0.0 | 100 |
| KGW 11-27 | 0.20 | + | 4.8×107 | 334 | 100 | 369 | 0.3 | 99.7 | n.t.e) | n.t. | n.t. |
| MYG 09-1 | 0.39 | + | 1.4×106 | 372 | 98.4 | 341 | 0.0 | 100 | 348 | 0.3 | 99.7 |
| MIE 94-3 | 0.39 | + | 1.9×106 | 385 | 13.0 | 363 | 0.0 | 100 | 370 | 0.0 | 100 |
| HKD 07-1 | 0.39 | + | 2.2×106 | 334 | 98.5 | 356 | 0.0 | 100 | 384 | 0.0 | 100 |
| SIG 06-8 | 0.39 | + | 2.4×106 | 375 | 99.7 | 383 | 0.3 | 99.7 | 360 | 0.0 | 100 |
| KGW 11-2 | 0.39 | + | 3.0×106 | 374 | 99.5 | 356 | 0.3 | 99.7 | n.t. | n.t. | n.t. |
| KGW 11-17 | 0.39 | + | 3.3×106 | 376 | 21.5 | 366 | 0.0 | 100 | n.t. | n.t. | n.t. |
| SIG 06-6 | 0.39 | + | 6.9×106 | 390 | 99.2 | 365 | 0.0 | 100 | 343 | 0.3 | 99.7 |
| SIG 09-3 | 0.39 | + | 7.0×106 | 386 | 99.2 | 360 | 0.0 | 100 | 358 | 0.0 | 100 |
| KGW 11-13 | 0.78 | + | 1.0×106 | 373 | 11.5 | 369 | 0.0 | 100 | n.t. | n.t. | n.t. |
| HKD 07-3 | 0.78 | + | 1.2×106 | 340 | 100 | 363 | 0.0 | 100 | 386 | 0.3 | 99.7 |
| HKD 07-2 | 0.78 | + | 3.5×106 | 357 | 99.4 | 357 | 0.0 | 100 | 369 | 0.0 | 100 |
| KGW 11-8 | 0.78 | + | 3.5×106 | 373 | 100 | 367 | 0.3 | 99.7 | n.t. | n.t. | n.t. |
| KGW 11-1 | 0.78 | + | 4.5×106 | 371 | 100 | 356 | 0.3 | 99.7 | n.t. | n.t. | n.t. |
| YMG 09-3 | 1.56 | — | 2.0×106 | 332 | 0 | 370 | 0.0 | — | 387 | 0 | — |
| SIG 09-6 | 3.13 | — | 1.1×106 | 374 | 0 | 379 | 0.0 | — | 363 | 0 | — |
| SIG 09-4 | 6.25 | — | 1.2×106 | 368 | 0 | 368 | 0.0 | — | 357 | 0 | — |
| Uninoculated | — | — | 378 | 0 | — | — | — | — | — | — | |
a) Detected by the bioassay of Nishijimas’ method. +: gibberellin producer, —: gibberellin non-producer. b) Dwarf rice cultivar, Tanginbozu was inoculated by dipping in the conidial suspension under vacuum condition. The volumetric ratio of seeds versus conidial suspension was 1 : 1. c) The percentage of diseased seedlings (elongated and dumping-off seedlings) in the total number of seedlings. d) Based on the percentage of diseased seedlings in untreated plot. e) n.t.: Not tested.
In the historical perspective, the prevalence of rice “Bakanae” disease was caused by the large-scale systems for raising rice seedlings8) and the development of isolates resistant to benzimidazole fungicides in Japan in the 1980s.9–12) From the late 1980s to the early 1990s, DMI fungicides such as prochloraz, triflumizole, pefurazoate and ipconazole were introduced as the alternative to benzimidazoles. Recently, isolates resistant to prochloraz were reported in Korea, although these isolates have no cross-resistance to other DMIs.13) The degradation of prochloraz by F. fujikuroi was indicated as the main mechanism of resistance.14) In addition, isolates less sensitive to pefurazoate15) were reported in Japan. Therefore, continuing investigation of the sensitivity of F. fujikuroi and efficacy of DMI fungicides should be necessary.
In Europe, isolates resistant to cyproconazole against wheat Septoria blight had not reported in 1993.16) However, in 2010, a decrease of efficacy of DMIs corresponding to mutation in CYP 51 was reported.17) These decreased efficacies of DMIs are considered to be caused by the “Directional selection.”
In the previous study of sensitivity to ipconazole on the former F. moniliforme, MIC values ranged from 0.10 to 6.25 mg/L with a peak at 0.39 and a shoulder peak at 3.13 mg/L.5) This result was reconfirmed with Ff complex group A (isolates randomly chosen from the previous study), although the shoulder peak at 3.13 mg/L was ambiguous in this study. MIC values of the Ff complex isolates, which were obtained after 1994 (group B), ranged from 0.2 to 6.25 mg/L and this range was within the range of previous study.
The species-diagnostic PCR-RFLP revealed that the former F. moniliforme isolates from rice consisted of F. fujikuroi and F. proliferatum. Furthermore, gibberellin producers and non-producers existed in F. fujikuroi isolates. The MIC range of gibberellin producers (0.10 to 0.78 mg/L) was lower than that of non-producers (0.78 to 6.25 mg/L), although they overlapped at 0.78 mg/L. The MIC values of F. proliferatum were relatively high (1.56 to 6.25 mg/L).
In some sensitivity studies of former F. moniliforme isolates to other DMIs, lower sensitivities of the gibberellin non-producers to DMIs were reported.18,19) The less-sensitive isolates of F. moniliforme in the previous studies could be the gibberellin non-producers of F. fujikuroi or F. proliferatum. It has been reported that some DMIs inhibit the gibberellin production of F. fujikuroi.2,18,19) Takenaka et al.20) reported that the less-sensitive mutants selected by pefurazoate showed low virulence to rice and low gibberellin production. They suggested the involvement of the enzyme component or factor for the demethylation of 24-methylenedihydrolanosterol in the pathway to ergosterol and kaurene oxidation steps in the pathway to gibberellins. This phenomenon implies that there is a relationship between the sensitivity of F. fujikuroi isolates to DMIs and their gibberellin production. However, to our knowledge, no report indicates the similarity of proteins or nucleotide sequence of 14α-demethylase and kaurene oxidase. Presumably, there are 2 groups (gibberellin produces and non-producers) in F. fujikuroi and their sensitivities to DMIs are originally different. Further investigation of the underlying mechanisms in the relationship between the sensitivity of DMIs and gibberellin production in F. fujikuroi may be necessary.
Most of Ff complex isolates from elongated stems of rice were gibberellin producers of F. fujikuroi. On the other hand, many gibberellin non-producers of F. fujikuroi were obtained from rice seeds. The comparison between all F. fujikuroi isolates in group B and group A does not seems to be proper when discussing the shift of sensitivity to ipconazole because the MIC values of gibberellin non-producers were higher than those of gibberellin producers. The comparison of gibberellin producers in group A and B may be appropriate when discussing the sensitivity in this case.
In this study, no symptoms of “Bakanae” disease were observed in seedlings whose seeds were inoculated with gibberellin non-producers (Table 2). The virulence of gibberellin non-producers to rice seedlings was also previously investigated by inoculation at the flowering stage and no “Bakanae” symptoms were observed until the harvest stage.21,22)
In the 200-fold dipping treatment of ipconazole 6% wettable powder, the deposit of ipconazole in seeds after water soaking and germination was 56 µg/g of seeds.2) Almost the same deposits in seeds were obtained by using other practical seed-treatment methods such as 20-fold 10 min. dipping, 0.5% of wetted dressing and 7.5-fold 3% seed weight spraying.2)
Since the start of practical use of ipconazole as a seed disinfectant, the sensitivity to ipconazole of virulent isolates (gibberellin producers) to ipconazole has been still stable for about 20 years. The practical dosage of ipconazole seed treatment showed stable efficacy against all virulent isolates with different sensitivities to ipconazole. No shift of sensitivity to ipconazole may contribute to the stable efficacy against “Bakanae” disease. It could be important for sustainable efficacy that the whole range of sensitivity in virulent F. fujikuroi isolates is covered with the practical dosage of ipconazole with a sufficient deposit in the treated seeds. Twenty years ago, it was suggested that the resistant development was not likely to occur because of the sufficient margins between the range of MIC values and the deposit of ipconazole in the treated seeds,5) and then our results in this study indicate that the suggestion seems to be correct.
We are deeply grateful to the staff of Kagawa prefectural Agriculture Research Station for kindly supplying the Ff complex isolates.