2016 Volume 41 Issue 4 Pages 133-144
2016 Volume 41 Issue 4 Pages 133-144
Thiocarbamate sulfoxides, which are the active forms of thiocarbamate herbicides, are quickly conjugated with glutathione and decomposed in soil. To achieve more potent and stable herbicidal activity, we previously developed a 5-{[(2,6-difluorophenyl)methoxy]methyl}-5-methyl derivative, which has a 4,5-dihydro-1,2-oxazole ring in place of the thiocarbamate to prevent conjugation and decomposition. Although the derivative showed pre-emergence herbicidal activity under flooded conditions, it displayed no herbicidal activity under upland conditions. In contrast, a 5-(methoxymethyl)-5-methyl derivative showed pre-emergence herbicidal activity against grass weeds under upland conditions. The aim of this study was to obtain a more potent compound with improved physicochemical properties for use as a pre-emergence upland herbicide via the structural optimization of a 3-{[(hetero)aryl]methanesulfonyl}-4,5-dihydro-1,2-oxazole as the core structure. In this way, we have developed the pre-emergence herbicide 3-{[5-(difluoromethoxy)-1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl]methanesulfonyl}-5,5-dimethyl-4,5-dihydro-1,2-oxazole, which has been named “pyroxasulfone.” This novel compound displayed excellent herbicidal activity against grass and broadleaf weeds under upland conditions with no phytotoxicity against crops.
3-{[5-(Difluoromethoxy)-1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl]methanesulfonyl}-5,5-dimethyl-4,5-dihydro-1,2-oxazole (pyroxasulfone, developmental code number KIH-485; Fig. 1) has been reported to exhibit excellent herbicidal activity against grass weeds and small-seeded broadleaf weeds with sufficient crop safety for corn, soybeans and wheat on pre-emergence application.1) In this paper, we describe the development of this herbicide, with a focus on structural optimization to enhance its herbicidal activities and improve its physicochemical properties.

The chemical control of grass weeds is important to ensure adequate yields from the cultivation of grains such as corn, wheat and rice. With typical pre-emergence herbicides, including thiobencarb and prosulfocarb (thiocarbamate types) and acetochlor, alachlor and metolachlor (chloroacetanilide types), an application rate of 1–4 kg of active ingredient per hectare (a.i./ha) is needed for sufficient grass-weed control. To develop sustainable agriculture that maintains agricultural production in harmony with the environment, dose reductions are needed.
Thiobencarb (Ia) (Alkyl=Et, X=4-Cl) (Fig. 2), which was developed as a rice herbicide by the Kumiai Chemical Industry Co., Ltd.,2) shows pre-emergence herbicidal activity against Echinochloa spp., Digitaria ciliaris and several other annual weeds in both upland and paddy fields. Herbicides such as thiobencarb, which are inhibitors of fatty acid and lipid biosynthesis,3,4) are classified into group N by the Herbicide Resistance Action Committee.5) The active forms of thiocarbamate herbicides (Ia) are believed to be the corresponding oxidative metabolites, thiocarbamate sulfoxides (Ib),6–9) which are rapidly conjugated with glutathione6–9) and decomposed in soil.7,8) To overcome this defect, we designed general template II with no carbonyl moiety by replacing the amide group with a heterocyclic ring to prevent rapid conjugation of these compounds with glutathione. For the heterocyclic ring, we selected 4,5-dihydro-1,2-oxazole, which is easily synthesized by a 1,3-dipolar cycloaddition reaction between a nitrile oxide and an olefin. Although a 4,5-dihydro-1,2-oxazole ring is a unique chemical structure for pre-emergence upland herbicides, many pesticides with this ring, e.g., III10,11) and IV,12,13) have been patented. We designed and synthesized 3-[(2,6-difluorophenyl)methanesulfonyl]-5-{[(2,6-difluorophenyl)methoxy]methyl}-5-methyl-4,5-dihydro-1,2-oxazole (V) as a novel pre-emergence herbicide.14) Compound V showed pre-emergence herbicidal activity against E. oryzicola at 250 g a.i./ha under flooded conditions but had no herbicidal activity against E. crus-galli (L.) Beauv. var. crus-galli (ECHCG) or Setaria viridis (L.) Beauv. (SETVI) at 1 kg a.i./ha under upland conditions. In contrast, compounds VI and VII showed pre-emergence herbicidal activity under upland conditions, compound VI showed herbicidal activity against ECHCG at 1 kg a.i./ha, and compound VII showed moderate herbicidal activity against ECHCG at 63 g a.i./ha and SETVI at 250 g a.i./ha.14)

Upland pre-emergence herbicides are applied to the soil surface and subsequently move downward into the soil with gravity. These herbicides can behave in a variety of different ways in soil, depending on whether they are dissolved in soil water, adsorbed or desorbed by the soil, or decomposed in the soil. For a compound to exhibit herbicidal efficacy in soil, it needs to form an herbicide-treated layer in close proximity to the weed seeds in soil. The distributions of alachlor and metolachlor have been examined using soil column chromatography, which is a common method for evaluating the mobility of herbicidal agents in soil. The results of that study revealed that alachlor exhibited a distribution pattern similar to that of metolachlor. The soil adsorption coefficient (Kd) values of alachlor and metolachlor have also been examined in nine soil types, and the results revealed that the Kd value of alachlor was similar to that of metolachlor.15) We believe that the Kd value of a herbicide is an important parameter for predicting its mobility in soil. With this in mind, we measured the Kd values of a series of compounds VIII and compared their mobility characteristics with those of chloroacetanilide herbicide, acetochlor, alachlor, and metolachlor.
In this study, we obtained a more potent compound with improved physicochemical properties as a pre-emergence herbicide under upland conditions through the structural optimization of compound VII derivatives having 3-{[(hetero)aryl]methanesulfonyl}-4,5-dihydro-1,2-oxazole (VIII) as the core structure.
The synthetic procedures used for pyroxasulfone (56) are detailed below. Starting materials were synthesized according to the schemes shown in Fig. 4.14,16–18) Compounds 1–55 and 57–62 were synthesized in a manner similar to that for 56 and have been disclosed previously in Refs. 14 and 17–19 and these patent families. Instrumental analysis and 1H NMR, HRMS and mp or nD20 data of the other compounds are given in the online supplementary materials.


NaSH (hydrate) (70%, 5.6 g, 70.0 mmol) was added to a solution of 5,5-dimethyl-3-(ethanesulfonyl)-4,5-dihydro-1,2-oxazole (6.7 g, 35.0 mmol) in DMF (50 mL) at room temperature. The mixture was stirred for 1 hr before the addition of K2CO3 (4.8 g, 35.0 mmol) and 4-(bromomethyl)-5-(difluoromethoxy)-1-methyl-3-(trifluoromethyl)-1H-pyrazole (10.8 g, 35.0 mmol), followed by stirring overnight at room temperature. The reaction mixture was poured into water and extracted with AcOEt. The resulting organic phase was washed successively with water and brine, dried over anhydrous MgSO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexane–AcOEt) to obtain 3-{[5-(difluoromethoxy)-1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl]methanesulfanyl}-5,5-dimethyl-4,5-dihydro-1,2-oxazole (7.3 g, 57.9%) as white crystals (mp: 39–40°C). 1H NMR δ (CDCl3): 1.42 (6H, s, 5,5-(CH3)2), 2.78 (2H, s, 4-CH2-), 3.81 (3H, s, pyrazole-1-CH3), 4.18 (2H, s, 3-SCH2-), 6.72 (1H, t, JH-F=72.0 Hz, pyrazole-5-OCHF2). HRMS m/z ([M+H]+): Calcd. for C12H15F5N3O2S: 360.0805, Found: 360.0798.
1.2. 3-{[5-(Difluoromethoxy)-1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl]methanesulfonyl}-5,5-dimethyl-4,5-dihydro-1,2-oxazole (56)mCPBA (70%, 12.5 g, 50.8 mmol) was added to a solution of 3-{[5-(difluoromethoxy)-1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl]methanesulfanyl}-5,5-dimethyl-4,5-dihydro-1,2-oxazole (7.3 g, 20.3 mmol) in CHCl3 (50 mL) with ice-cooling. The mixture was stirred for 1 hr and then stirred at room temperature overnight. The reaction mixture was poured into water and extracted with CHCl3. The resulting organic phase was washed successively with aq. NaHSO3, aq. NaHCO3, water, and brine, dried over anhydrous MgSO4, and concentrated in vacuo to obtain 3-{[5-(difluoromethoxy)-1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl]methanesulfonyl}-5,5-dimethyl-4,5-dihydro-1,2-oxazole (6.4 g, 80.6%) as a white powder (mp: 129–130°C.). 1H NMR δ (CDCl3): 1.52 (6H, s, 5,5-(CH3)2), 3.11 (2H, s, 4-CH2-), 3.88 (3H, s, pyrazole-1-CH3), 4.60 (2H, s, 3-SO2CH2-), 6.83 (1H, t, JH-F=71.8 Hz, pyrazole-5-OCHF2). HRMS m/z ([M+H]+): Calcd. for C12H15F5N3O4S: 392.0698, Found: 392.0697.
2. Measurement of the log P and Kd valuesThese experiments were conducted as reported in Ref. 20.
3. Formulation of test compoundsTen parts of compound VIII were mixed with 0.5 part polyoxyethylene octylphenyl ether, 0.5 part the sodium salt of 2-naphthalenesulfonic acid-formalin condensate, 20 parts diatomaceous earth, and 69 parts clay. The mixture was mixed and pulverized to obtain a wettable powder.
4. Pre-emergence herbicidal activity testPlastic pots (336 cm2) were filled with upland soil. The soil texture of the upland soil was a sandy loam with 0.66% organic matter and a pH of 6.97. Seeds of Zea mays L. (ZEAMX), Glycine max L. (GLXMA), E. crus-galli var. crus-galli (ECHCG) and S. viridis (SETVI), along with Chenopodium album L. (CHEAL) and Abutilon theophrasti Medik. (ABUTH) in some cases, were sown 1 cm below the top of each pot and covered with the same soil. A wettable powder was diluted with water and sprayed uniformly on the soil surface using a small sprayer at a spray volume of 1000 L/ha to give the applied amount of each active ingredient shown in Tables 1–8 without repetition. The plants were then grown in a greenhouse, and herbicidal activity and phytotoxicity of each compound were evaluated by visual observation of the symptoms of treated plants compared with untreated controls at 21 days after application. Herbicidal activity and phytotoxicity were assessed on a scale of 0 (no activity) to 10 (complete kill). If a compound exhibited an index of herbicidal activity of 9 or 10 against the tested weeds, it was deemed to have sufficient herbicidal activity. If the index of phytotoxicity against crops was 2 or lower, the agent was considered to be safe for crops.
5. Field trialsField experiments were conducted in an experimental field at the Life Science Research Institute of the Kumiai Chemical Industry Co., Ltd. (Kakegawa, Shizuoka), in April 2002. The soil texture of the experimental field was a clay loam with 0.92–1.18% organic matter and a pH of 6.09–6.29. Seeds of weeds ECHCG, SETVI and Amaranthus retroflexus (AMARE) were sown in the test area and immediately mixed with soil on April 24. Seeds of ZEAMX were planted on April 25. The plot was 2 m2, arranged in a randomized complete block design, and replicated three times. Formulations of compounds 45 and 56 were diluted with water and applied immediately at a spray volume of 500 L/ha to give the applied amount of each active ingredient shown in Table 9 after ZEAMX planting, with the commercial herbicide Dual II Magnum (S-metolachlor 82.4%) used as a positive control. Herbicidal activity and phytotoxicity were assessed visually 26 days after application on a scale of 0 to 100, with 0 representing no phytotoxicity or efficacy and 100 corresponding to crop death or complete weed control.
After synthesizing compounds 1–9 with alkyl substituents at the 4- and 5-positions of the 4,5-dihydro-1,2-oxazole moiety, we examined their herbicidal activities against ECHCG and SETVI, which are critical weeds in ZEAMX and GLXMA cultivation,21) and their phytotoxicities toward ZEAMX and GLXMA (Table 1). Compounds 1–3 and 7 exhibited sufficient herbicidal activity against ECHCG; compound 6 also acted as an effective herbicide but was slightly less potent. This result suggests that the possession of sufficient herbicidal activity against ECHCG requires that substituents R1 and R2 at the 5-position should independently be a hydrogen or an alkyl group with two or fewer carbon atoms, while substituent R3 at the 4-position should be a hydrogen atom. Against SETVI, in contrast, compounds 3 and 6 showed sufficient herbicidal activity, suggesting that a methyl or an ethyl for R1, a methyl group for R2, and a hydrogen atom for R3 are required for sufficient herbicidal activity against SETVI. Although compounds 3, 5 and 6 were slightly phytotoxic to ZEAMX, they were considered to be safe for crops. Taking all of these results together, compounds 3 and 6 showed potent herbicidal activity against weeds with adequate safety for crops. Compared with compound 6, compound 3 has two synthetic advantages: it has no asymmetric carbon atom on the 4,5-dihydro-1,2-oxazole ring and its synthetic precursor, 2-methylpropene, is commercially available in large quantities. We therefore modified the substituted benzyl moiety of compound 3, which has a 5,5-dimethyl-4,5-dihydro-1,2-oxazole ring as an optimized structure of 4,5-dihydro-1,2-oxazole moiety, to further improve pre-emergence herbicidal efficacy under upland conditions.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | R1 | R2 | R3 | g a.i./ha | Herbicidal activity and phytotoxicitya) | |||
| ECHCGb) | SETVIb) | ZEAMXb) | GLXMAb) | |||||
| 1 | H | H | H | 63 | 9 | 0 | 0 | 0 |
| 2 | Me | H | H | 63 | 9 | 6 | 0 | 0 |
| 3 | Me | Me | H | 63 | 9 | 9 | 1 | 0 |
| 4 | Me | H | Me | 63 | 3 | 4 | 0 | 0 |
| 5 | Me | Me | Me | 63 | 4 | 3 | 1 | 0 |
| 6 | Et | Me | H | 63 | 8 | 9 | 2 | —c) |
| 7 | Et | Et | H | 63 | 9 | 6 | 0 | 0 |
| 8 | Pr | Me | H | 63 | 1 | 1 | 0 | 0 |
| 9 | i-Pr | Me | H | 63 | 0 | 0 | 0 | 0 |
| VII | 250 | 9 | 7 | 0 | 2 | |||
| 63 | 5 | 0 | 0 | —c) | ||||
a) Visual assessment was conducted 21 days after pre-emergence application. b) ECHCG: Echinochloa crus-galli (L.) Beauv. var. crus-galli, SETVI: Setaria viridis (L.) Beauv., ZEAMX: Zea mays, GLXMA: Glycine max. c) Not tested.
We tested benzyl-substituted compounds 10–18 having three types of substituents (-Cl, -Me and -OMe) at the 2-, 3- or 4-position of the benzene ring (Table 2). The 2-substituted compounds 11, 14 and 17 exhibited herbicidal activities equivalent to or slightly greater than those of non-substituted compound 10 at 63 g a.i./ha, whereas the herbicidal activities of the 3- and 4-substituted compounds 12, 13 and 16 were less than that of compound 10. This result suggests that the substituent should be introduced at the 2-position on the benzene ring rather than at the other positions and that the steric effect of the substituent at the 2-position is concerned with its herbicidal activity. In comparison with the herbicidal activity of compound 3 and those of compounds 10, 11, 14 and 17, this result suggests that other factors, such as an electron-withdrawing or an inductive effect of the substituent, are concerned with their herbicidal activity. We thus focused on further modification of the 2-substituent.
 | ||||||
|---|---|---|---|---|---|---|
| No. | X | g a.i./ha | Herbicidal activity and phytotoxicitya) | |||
| ECHCGb) | SETVIb) | ZEAMXb) | GLXMAb) | |||
| 10 | H | 63 | 8 | 7 | 2 | 0 |
| 16 | 1 | 2 | 0 | 0 | ||
| 11 | 2-Cl | 63 | 7 | 6 | 0 | 0 |
| 16 | 1 | 0 | 0 | 0 | ||
| 12 | 3-Cl | 63 | 1 | 3 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | ||
| 13 | 4-Cl | 63 | 3 | 7 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | ||
| 14 | 2-Me | 63 | 9 | 8 | 0 | 0 |
| 16 | 2 | 0 | 0 | 0 | ||
| 15 | 3-Me | 63 | 6 | 7 | 0 | 0 |
| 16 | 2 | 0 | 0 | 0 | ||
| 16 | 4-Me | 63 | 4 | 2 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | ||
| 17 | 2-OMe | 63 | 8 | 8 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | ||
| 18 | 3-OMe | 63 | 9 | 6 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | ||
| 3 | 2,6-F2 | 63 | 9 | 9 | 1 | 0 |
| 16 | 7 | 4 | —c) | 0 | ||
a), b), c) as shown in Table 1.
Various substituents such as halogens (-F, -Br and -I), ethyl, ethoxy, difluoromethoxy, trifluoromethoxy, trifluoromethyl, nitro, and cyano groups, were introduced at the 2-position of the benzene ring (Table 3). Compounds 21 and 23–26 exhibited sufficient herbicidal activities against ECHCG and SETVI at 63 g a.i./ha. In particular, the activities of compounds 24 and 25, which respectively contained a difluoromethoxy and trifluoromethoxy group, were excellent even at 16 g a.i./ha. Compounds 24 and 25 were phytotoxic to ZEAMX at 63 g a.i./ha. Compound 26 showed no phytotoxicity toward either crop, although its herbicidal activity was slightly lower than those of compounds 24 and 25. This result suggests that a hydrophobicity and an electron-withdrawing effect of the alkoxy group at the 2-position are very important factors in exhibiting excellent herbicidal activity. Considering these results, we introduced an additional substituent at another position on the benzene ring of compounds 24 and 26 to reduce the phytotoxicity of the former with its excellent herbicidal activity and to improve the herbicidal activity of the latter with its non-phytotoxicity.
 | ||||||
|---|---|---|---|---|---|---|
| No. | X | g a.i./ha | Herbicidal activity and phytotoxicitya) | |||
| ECHCGb) | SETVIb) | ZEAMXb) | GLXMAb) | |||
| 19 | 2-F | 63 | 8 | 7 | 0 | 0 |
| 16 | 2 | 2 | 0 | 0 | ||
| 20 | 2-Br | 63 | 9 | 7 | 0 | 0 |
| 16 | 2 | 1 | 0 | 0 | ||
| 21 | 2-I | 63 | 9 | 9 | 1 | 0 |
| 16 | 2 | 3 | 0 | 0 | ||
| 22 | 2-Et | 63 | 8 | 8 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | ||
| 23 | 2-OEt | 63 | 10 | 9 | 1 | 1 |
| 16 | 7 | 8 | 0 | 0 | ||
| 24 | 2-OCHF2 | 63 | 10 | 10 | 5 | —c) |
| 16 | 9 | 9 | 0 | 0 | ||
| 25 | 2-OCF3 | 63 | 10 | 10 | 3 | 0 |
| 16 | 9 | 8 | 0 | 0 | ||
| 26 | 2-CF3 | 63 | 9 | 9 | 0 | 0 |
| 16 | 6 | 6 | 0 | 0 | ||
| 27 | 2-NO2 | 63 | 8 | 8 | 2 | 0 |
| 16 | 4 | 0 | 0 | 0 | ||
| 28 | 2-CN | 63 | 8 | 8 | 0 | 0 |
| 16 | 3 | 1 | 0 | 0 | ||
| 11 | 2-Cl | 63 | 7 | 6 | 0 | 0 |
| 16 | 1 | 0 | 0 | 0 | ||
| 14 | 2-Me | 63 | 9 | 8 | 0 | 0 |
| 16 | 2 | 0 | 0 | 0 | ||
| 17 | 2-OMe | 63 | 8 | 8 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | ||
a), b), c) as shown in Table 1.
We examined compounds 29–40, in which a chlorine, fluorine, methyl, or methoxy group was introduced on the benzene ring of compound 24 to reduce its phytotoxicity (Table 4). Although compound 34 exhibited excellent herbicidal activities equivalent to those of compound 24, its phytotoxicity for ZEAMX was even worse than that of compound 24. The herbicidal activities of the compounds substituted at the 6-position, except for a fluorine, were inferior to those of compound 24. This result suggests that the steric space at the 6-position is limited. The introduction of the hydrophobic substituents at the 5-position reduced their herbicidal activities slightly, but their phytotoxicities for ZEAMX were the same or improved. Based on the herbicidal activities of compounds 29 and 30, the introduction of a chlorine atom at the 3- or 4-position led to a reduction of herbicidal activities at 16 g a.i./ha. Considering these results, we found that it is possible to improve herbicidal activity with the introduction of a fluorine at the 6-position and reduce phytotoxicity with the introduction of a hydrophobic substituent at the 5-position.
 | ||||||
|---|---|---|---|---|---|---|
| No. | Y | g a.i./ha | Herbicidal activity and phytotoxicitya) | |||
| ECHCGb) | SETVIb) | ZEAMXb) | GLXMAb) | |||
| 29 | 3-Cl | 63 | 9 | 7 | 0 | 0 |
| 16 | 1 | 1 | 0 | 0 | ||
| 30 | 4-Cl | 63 | 9 | 9 | 0 | 0 |
| 16 | 2 | 3 | 0 | 0 | ||
| 31 | 5-Cl | 63 | 10 | 9 | 5 | 0 |
| 16 | 9 | 7 | 0 | 0 | ||
| 32 | 6-Cl | 63 | 9 | 9 | 1 | 0 |
| 16 | 5 | 3 | 0 | 0 | ||
| 33 | 5-F | 63 | 10 | 9 | 3 | 0 |
| 16 | 9 | 8 | 0 | 0 | ||
| 34 | 6-F | 63 | 10 | 10 | 9 | 6 |
| 16 | 10 | 9 | 5 | 0 | ||
| 35 | 4-Me | 63 | 7 | 7 | 0 | 0 |
| 16 | 6 | 2 | 0 | 0 | ||
| 36 | 5-Me | 63 | 10 | 9 | 0 | 0 |
| 16 | 9 | 7 | 0 | 0 | ||
| 37 | 6-Me | 63 | 10 | 9 | 0 | 0 |
| 16 | 9 | 6 | 0 | 0 | ||
| 38 | 4-OMe | 63 | 7 | 9 | 0 | 0 |
| 16 | 2 | 3 | 0 | 0 | ||
| 39 | 5-OMe | 63 | 10 | 8 | 0 | 0 |
| 16 | 7 | 4 | 0 | 0 | ||
| 40 | 6-OMe | 63 | 9 | 8 | 0 | 0 |
| 16 | 0 | 1 | 0 | 0 | ||
| 24 | H | 63 | 10 | 10 | 5 | —c) |
| 16 | 9 | 9 | 0 | 0 | ||
a), b), c) as shown in Table 1.
We then introduced the substituents such as a chlorine, fluorine, or methyl group, at the 5- and/or 6-position(s) of the benzene ring of compound 26 and evaluated the efficacy of the resulting compounds (Table 5). Compounds 41, 42 and 44 showed higher herbicidal activities than compound 26 with no increase in phytotoxicity toward ZEAMX at 63 and 16 g a.i./ha. Although compound 43 showed great herbicidal activity, it exhibited severe phytotoxicity toward ZEAMX and GLXMA. In contrast, compound 45 showed sufficient herbicidal activity against SETVI at 16 g a.i./ha, with low phytotoxicity toward ZEAMX and GLXMA even at 250 g a.i./ha. This result suggests that compound 45 is the best novel pre-emergence upland herbicide candidate among compounds in which the aryl portion of VIII is a benzene ring.
 | ||||||
|---|---|---|---|---|---|---|
| No. | Y | g a.i./ha | Herbicidal activity and phytotoxicitya) | |||
| ECHCGb) | SETVIb) | ZEAMXb) | GLXMAb) | |||
| 41 | 5-Cl | 250 | 10 | 10 | 6 | 6 |
| 63 | 10 | 10 | 0 | 0 | ||
| 16 | 7 | 9 | 0 | 0 | ||
| 42 | 5-F | 250 | 10 | 10 | 8 | 0 |
| 63 | 10 | 9 | 0 | 0 | ||
| 16 | 7 | 7 | 0 | 0 | ||
| 43 | 6-F | 250 | 10 | 10 | 9 | 4 |
| 63 | 9 | 10 | 8 | 2 | ||
| 16 | 9 | 9 | 4 | 1 | ||
| 44 | 5-Me | 250 | 10 | 10 | 4 | 0 |
| 63 | 10 | 9 | 0 | 0 | ||
| 16 | 9 | 7 | 0 | 0 | ||
| 45 | 5-Cl-6-F | 250 | 10 | 10 | 1 | 1 |
| 63 | 10 | 10 | 0 | 0 | ||
| 16 | 5 | 9 | 0 | 0 | ||
| 26 | H | 250 | 10 | 9 | 7 | 0 |
| 63 | 9 | 9 | 0 | 0 | ||
| 16 | 6 | 6 | 0 | 0 | ||
a), b) as shown in Table 1.
Based on the physicochemical properties of compound 45 and several other key compounds described in section 2, we synthesized compounds 46–51 containing a trifluoromethyl-substituted heteroaryl ring in place of the trifluoromethyl-substituted benzene ring of compound 45 to reduce the log P value and to enhance the herbicidal activity as a pre-emergence upland herbicide. We evaluated the herbicidal activities of these compounds against four kinds of weeds, including two broadleaf weeds such as CHEAL and ABUTH, as well as their phytotoxicities compared with those of compound 45 (Table 6). Among them, only compound 46 showed sufficient herbicidal activities against CHEAL at 16 g a.i./ha in addition to grass weeds, but it was phytotoxic to ZEAMX. We next replaced the chlorine at the 5-position of the pyrazole ring of compound 46 to reduce the phytotoxicity.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Q | g a.i./ha | Herbicidal activity and phytotoxicitya) | |||||
| ECHCGb) | SETVIb) | CHEALd) | ABUTHd) | ZEAMXb) | GLXMAb) | |||
| 46 |  | 63 | 10 | 10 | 10 | 6 | 7 | 0 |
| 16 | 8 | 9 | 10 | 0 | 1 | 0 | ||
| 47 |  | 63 | 10 | 9 | 0 | 0 | 0 | 0 |
| 16 | 7 | 6 | 0 | 0 | 0 | 0 | ||
| 48 |  | 63 | 10 | 8 | 2 | 0 | 0 | 0 |
| 16 | 8 | 1 | 0 | 0 | 0 | 0 | ||
| 49 |  | 63 | 10 | 8 | 5 | 0 | 0 | 0 |
| 16 | 10 | 2 | 0 | 0 | 0 | 0 | ||
| 50 |  | 63 | 9 | 7 | 0 | 0 | 0 | 0 |
| 16 | 7 | 2 | 0 | 0 | 0 | 0 | ||
| 51 | 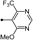 | 63 | 10 | 10 | 0 | 0 | 0 | 0 |
| 16 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| 3 |  | 63 | 9 | 9 | 0 | 3 | 1 | 0 |
| 16 | 7 | 4 | 0 | 0 | —c) | 0 | ||
| 45 |  | 63 | 10 | 10 | 0 | 4 | 0 | 0 |
| 16 | 5 | 9 | 0 | 0 | 0 | 0 | ||
a), b), c) as shown in Table 1. d) CHEAL: Chenopodium album, ABUTH: Abutilon theophrasti.
We examined compounds 52–58 that respectively have a fluorine, methyl, methoxy, ethoxy, difluoromethoxy, cyano and methanesulfonyl at the 5-position of the pyrazole ring. Among them, only compound 56 with the 5-difluoromethoxy group showed herbicidal activities equivalent to or greater than those of compound 46 against all tested weeds. Compound 56 exhibited sufficient herbicidal activity against ABUTH and no phytotoxicity toward ZEAMX and GLXMA at 63 g a.i./ha.
1.7. Evaluation of substituents at the 1-position of the pyrazole ring of compound 56Finally, we examined compounds 59–62 that were substituted by ethyl, isopropyl, tert-butyl or propyl groups at the 1-position of their pyrazole ring compared with the methyl group in compound 56 (Table 8). The herbicidal activities against SETVI of compounds 59–62 were slightly lower than those of compound 56. Only compound 56 showed sufficient herbicidal activities against ECHCG, SETVI, CHEAL and ABUTH at 63 g a.i./ha. Compounds 56 and 62 showed no phytotoxicities toward ZEAMX and GLXMA at 63 g a.i./ha. This result suggested that compound 56 was the best novel pre-emergence upland herbicide candidate among compounds in which the aryl portion of VIII was a pyrazole ring.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | R4 | g a.i./ha | Herbicidal activity and phytotoxicitya) | |||||
| ECHCGb) | SETVIb) | CHEALd) | ABUTHd) | ZEAMXb) | GLXMAb) | |||
| 52 | F | 63 | 10 | 8 | 1 | 0 | 6 | 0 |
| 16 | 9 | 6 | 0 | 0 | 0 | 0 | ||
| 53 | Me | 63 | 10 | 10 | 6 | 0 | 4 | 0 |
| 16 | 10 | 9 | 0 | 0 | 0 | 0 | ||
| 54 | OMe | 63 | 10 | 10 | 10 | 1 | 6 | 0 |
| 16 | 2 | 1 | 0 | 0 | 0 | 0 | ||
| 55 | OEt | 63 | 9 | 9 | 6 | 3 | 6 | 0 |
| 16 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| 56 | OCHF2 | 63 | 10 | 10 | 10 | 10 | 0 | 0 |
| 16 | 10 | 10 | 8 | 1 | 0 | 0 | ||
| 57 | CN | 63 | 10 | 10 | 10 | 2 | 6 | 0 |
| 16 | 10 | 9 | 0 | 0 | 0 | 0 | ||
| 58 | SO2Me | 63 | 10 | 10 | 3 | 0 | 6 | 0 |
| 16 | 10 | 8 | 0 | 0 | 0 | 0 | ||
| 46 | Cl | 63 | 10 | 10 | 10 | 6 | 7 | 0 |
| 16 | 8 | 9 | 10 | 0 | 1 | 0 | ||
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | R5 | g a.i./ha | Herbicidal activity and phytotoxicitya) | |||||
| ECHCGb) | SETVIb) | CHEALd) | ABUTHd) | ZEAMXb) | GLXMAb) | |||
| 59 | Et | 63 | 10 | 10 | 8 | 6 | 4 | 0 |
| 16 | 10 | 9 | 8 | 1 | 0 | 0 | ||
| 60 | iPr | 63 | 10 | 10 | 10 | 5 | 3 | 0 |
| 16 | 9 | 9 | 2 | 0 | 0 | 0 | ||
| 61 | tBu | 63 | 9 | 9 | 5 | 3 | 0 | 1 |
| 16 | 8 | 6 | 0 | 0 | 0 | 0 | ||
| 62 | Pr | 63 | 10 | 10 | 10 | 3 | 0 | 0 |
| 16 | 8 | 9 | 10 | 0 | 0 | 0 | ||
| 56 | Me | 63 | 10 | 10 | 10 | 10 | 0 | 0 |
| 16 | 10 | 10 | 8 | 1 | 0 | 0 | ||
The log P and soil adsorption coefficient (Kd) values of compounds 3, 24, 31, 45, 46 and 56 were measured to evaluate their mobility in the soil and potential as pre-emergence upland herbicides. These compounds were selected for evaluation because they showed sufficient herbicidal activity or contained key structural modifications compared with chloroacetanilide herbicides acetochlor, alachlor, and metolachlor (Fig. 5). The Kd values of the chloroacetanilide herbicides were very similar to each other, around 5, with log P values in the range of 3–4. The Kd values of compounds 3 and 24 were close to those of the chloroacetanilide herbicides despite the fact they had much lower log P values. In contrast, the Kd value of compound 45 was about three times higher than those of the chloroacetanilide herbicides. Compounds 46 and 56, which had pyrazole rings in place of the benzene ring of compound 45, had lower Kd values than that of compound 45 even though all three had similar hydrophobic substituents. The Kd values of compounds 46 and 56 were also very close to those of the chloroacetanilide herbicides with much lower log P values than compound 45 and the chloroacetanilide herbicides. These results therefore suggest that compounds 3, 24, 46 and 56 would behave in a manner similar to that of the chloroacetanilide herbicides in soil.

The herbicidal activities and phytotoxicities of compounds 45 and 56 were examined in the field (Table 9). Compound 45 exhibited no phytotoxicity toward ZEAMX at any tested application dose. However, compound 56 exhibited slight phytotoxicity at 250 g a.i./ha. Compounds 45 and 56 showed potent herbicidal activities against ECHCG, SETVI and AMARE at 250 g a.i./ha. Compound 56 also showed excellent herbicidal activities against the same weeds at 125 g a.i./ha. The herbicidal activities of compound 56 were greater than those of compound 45 at 125 and 63 g a.i./ha, which was expected on the basis of the relative Kd values of the two compounds and their herbicidal activities during pot trials.
| No. | g a.i./ha | Herbicidal activity and phytotoxicity | |||
|---|---|---|---|---|---|
| ECHCGb) | SETVIb) | AMAREe) | ZEAMXb) | ||
| 45 | 250 | 91 | 95 | 98 | 0 |
| 125 | 78 | 80 | 67 | 0 | |
| 63 | 48 | 45 | 20 | 0 | |
| 56 | 250 | 97 | 98 | 100 | 2 |
| 125 | 96 | 97 | 95 | 0 | |
| 63 | 86 | 87 | 79 | 0 | |
| S-metolachlor | 2140 | 99 | 99 | 100 | 1 |
| 1070 | 98 | 98 | 99 | 0 | |
b) as shown in Table 1. e) AMARE: Amaranthus retroflexus
The dissipation half-life (DT50) values of compound 56 and S-metolachlor on three field trials were provided by Westra et al.23) The DT50 values of compound 56 were higher than those of S-metolachlor under the same field conditions. This result suggests that compound 56 is more stable than S-metolachlor in soil.
In conclusion, compound 56 showed the highest herbicidal activity of all of the compounds tested in the current study against grass and broadleaf weeds, exhibited excellent safety for crops, had proper physicochemical properties as a pre-emergence upland herbicide and showed high stability in soil as a pre-emergence upland herbicide. Compound 56 has been named “pyroxasulfone” in accordance with the International Organization for Standardization and has been launched as a pre-emergence upland herbicide for use with corn, soybeans, cotton and wheat by the Kumiai Chemical Industry Co., Ltd., and the Ihara Chemical Industry Co., Ltd.
The authors thank the staff members of the research and development divisions of the K-I Chemical Research Institute Co., Ltd., the Kumiai Chemical Industry Co., Ltd. and the Ihara Chemical Industry Co., Ltd. for this research. The authors also thank the anonymous reviewers for their helpful suggestions that improved this paper.