2021 年 46 巻 1 号 p. 43-52
2021 年 46 巻 1 号 p. 43-52
Chitin deacetylase (CDA) is a key enzyme involved in the modification of chitin and plays critical roles in molting and pupation, which catalyzes the removal of acetyl groups from N-acetyl-D-glucosamine residues in chitin to form chitosan and release acetic acid. Defects in the CDA genes or their expression may lead to stunted insect development and even death. Therefore, CDA can be used as a potential pest control target. However, there are no effective pesticides known to target CDA. Although there has been some exciting research progress on bacterial or fungal CDAs, insect CDA characteristics are less understood. This review summarizes the current understanding of insect CDAs, especially very recent advances in our understanding of crystal structures and the catalytic mechanism. Progress in developing small-molecule CDA inhibitors is also summarized. We hope the information included in this review will help facilitate new pesticide development through a novel action mode, such as targeting CDA.

The enzyme chitin deacetylase (CDA; EC 3.5.1.41) hydrolyzes the acetamido group in the N-acetylglucosamine units of chitin, which generates the glucosamine unit chitosan and acetic acid (AcOH) (Fig. 1).1) Chitin is a linear homopolymer of β-1,4-glycosidically linked N-acetylglucosamine (GlcNAc) residues. It forms crystalline fibers and is insoluble in aqueous solvents, while chitosans are soluble at a slightly acidic pH.2) Deacetylation can alter the physical properties of the polymer to enhance solubility and flexibility and confers a positive charge at a neutral pH.3) CDAs are metalloproteins that belong to an extracellular chitin-modifying enzyme family, carbohydrate esterase family 4 (CE-4), as shown in the carbohydrate active enzymes (CAZY) database (http://www.cazy.org).4) There are several other members in this family, including the NodB protein (EC 3.5.1.-)5) and peptidoglycan deacetylase (EC 3.1.1.-).6,7) Rhizobial NodB is involved in de-N-acetylating the nonreducing end GlcNAc from short chito-oligosaccharides8); peptidoglycan deacetylases can modify bacterial cell wall peptidoglycan by de-N-acetylation of either the N-acetylmuramic acid (MurNAc) or GlcNAc residues of the disugar repeats.

CDAs are widely distributed in protozoa, diatoms, bacteria, fungi, nematodes, and insects but are not present in humans or plants.9–14) As a structural polysaccharide, chitin is an important component of the insect epidermis, trachea, peritrophic membrane, and other organs.15–18) The metabolic disorder of chitin components in insects can lead to abnormal life processes and even death.19) There are multiple genes in insects that encode CDA; they play a critical role in molting, pupation, and chitin modification. DcCDA3 from Diaphorina citri is suggested to play an important role in the immune response.20) The SpCDA1 gene in Stegobium paniceum is essential for successful larval–pupal transition.21) Locusta migratoria with reduced LmCDA2 activity died at molting.22) The LsCDA1 gene in Lasioderma serricorne is suggested to be indispensable for larval–pupal and pupal–adult molts,23) and RNAi-aided knockdown of two LdCDA2 variants in Leptinotarsa decemlineata resulted in retardation of larval growth, reduced chitin contents in the larvae, and abnormal morphology followed by insect death.24) Taken together, these severe phenotypes indicate that CDA is a potential pest control target. Considering that CDA is not contained in humans or plants, the research on CDA inhibitors will help to develop green biopesticides.
Recent advances have revealed the unique structural and biochemical features of insect CDAs, which provide important information for the development of insect-specific inhibitors. In this regard, this review summarizes the current understanding of insect CDAs, especially very recent advances in our understanding of crystal structures and the catalytic mechanism. Progress in developing small-molecule CDA inhibitors is also summarized. The information included in this review may help facilitate new pesticide development through a novel action mode, such as targeting CDA.
Insect CDAs are numerous and widely distributed in various tissues and organs.16–18) There are differences in domain composition, tissue specificity, and physiological function among them. A comparative analysis of CDA gene families in several insect species with fully sequenced genomes, including Diptera, Coleoptera, Hymenoptera, and Lepidoptera, revealed that the number of CDA genes varies with the species. Based on amino acid sequence similarity, insect CDAs are classified into five groups, I–V (Fig. 2).25) All groups CDAs contain a conserved NodB domain, while they also have unique structural elements.26) Based on the specific structural elements of different groups, the corresponding inhibitor structures may be varied.

Group I CDAs: All group I CDAs have a chitin-binding peritrophin-A domain (CBD), a low-density lipoprotein receptor class A domain (LDLa), and a CDA catalytic domain (CAD). In each species, there are usually two CDAs of group I, CDA1 and CDA2 (the amino acid sequence identity between CDA1s and CDA2s is approximately 60%). Interference with this group of CDA genes can lead to molting disorders and death, as well as abnormal development of the trachea, fin sheath, and joint.16,18,22) To date, studies on the function of group I CDAs are most numerous. In Drosophila melanogaster, two group I genes, DmSerp (DmCDA1) and DmVerm (DmCDA2), are required for normal tracheal tube development and morphology.16) D. melanogaster mutants that lack either of the two genes named serpentine (serp) and vermform (verm) exhibited excessively long and tortuous embryonic tracheal tubes. Arakane et al. carried out a detailed RNA interference (RNAi) analysis of nine Tribolium castaneum CDA genes,19) and the results showed that TcCDA1 or TcCDA2 dsRNA injection will affect all types of molting. The RNAi of TcCDA2a affected femoral-tibial joint movement, whereas the RNAi of dsTcCDA2b resulted in elytra with crinkled and rough dorsal surfaces. Depletion of either TcCDA1 or TcCDA2 results in a disorganized cuticle filled with smaller fibers without the normally stacked laminae, indicating that TcCDAs play a critical role in the elongation/organization of smaller nanofibers into longer fibers.27) HvCDA1 and HvCDA2 genes in Heortia vitessoides are suggested to play important roles in the larval–pupal and pupal–adult transitions.28) These results indicate that group I CDAs play critical roles in the structural integrity of the cuticular chitin laminae and chitin fibers of the tracheal tube.
Group II CDAs: Group II CDAs also have a CBD, LDLa, and CAD. Group II only includes CDA3. However, like group I CDAs, CDA3s also possess a single copy of each of the three domains, but the overall amino acid sequence has only about 38% identity with CDA1s and CDA2s. However, no abnormal phenotype was found in the RNAi study of this kind of CDA.
Group III CDAs: Group III includes only one CDA, CDA4. Group III (CDA4s) has a single copy of the CBD and CDA catalytic domains but lacks an LDLa domain. Currently, there are few studies on the location and function of group III CDAs. The results of an RNAi study on the Nilaparvata lugens CDA17) revealed that the RNAi of NlCDA4 led to a lethal phenotype and high mortality caused by abnormal shedding, which indicates a possible role in molting.
Group IV CDAs: Group IV includes CDA5s that, like CDA4s, each possess a single CBD and a single CDA catalytic domain. These two domains, however, are connected by a long Ser/Thr/Pro/(Gln)-rich linker, which results in CDA5s being the largest CDA proteins. There have been very few studies on group IV; however, Xi et al. found that CDAs from both groups I and IV are involved in functions associated with molting in the hemimetabolous insect N. lugens.17)
Group V CDAs: Group V CDAs, which include CDAs 6–9, have only one catalytic domain structure. These CDAs are specifically expressed in the midgut and are also known as midgut CDAs.22) No adverse phenotypic effects were observed when dsRNAs for the T. castaneum gut-specific CDAs, TcCDAs 6–9, were co-injected during the young larval stage.19) The absence of RNAi effects revealed that the gut CDAs may not be essential for survival; however, they may still contribute to insect defense against pathogens. Jakubowska et al.29) observed that one of the group V CDA genes (CDA9) from Helicoverpa armigera (HaCDA5a) was downregulated by baculovirus infection in larvae. Recombinant baculoviruses that express this protein have shown significant increases in kill speed and pathogenicity against larvae in different Spodoptera spp., two parameters that are highly used in baculovirus-based biopesticides. Incubation of the peritrophic membrane (PM) from Spodoptera frugiperda with recombinant HaCDA5a increased PM permeability in a concentration-dependent manner. These observations indicate that the group V CDAs may play a role in determining PM structure/morphology or permeability.
Determination of enzymatic activity was a major challenge in insect CDA studies.30) Most studies to determine insect CDA activity used artificial substrates. Toprak et al.31) reported the activity of a Mamestra configurata CDA (McCDA1) toward colloidal chitin using an in-gel assay that detects chitosan formation. Zhong et al.12) used a procedure for measuring CDA activity in an assay based on Srinivasan’s patent, in which the CDA catalyzes p-nitroacetanilide conversion to p-nitroaniline; they measured the CDA activity of a Bombyx mori CDA (BmCDA7) and found that recombinant BmCDA7 was active. Recently, Liu et al. first reported the enzymatic activities of insect CDAs toward their physiological substrates.32) The results indicated that BmCDA7 and BmCDA8 were active toward peritrophic membrane (PM) chitin, while BmCDA6 was inactive. Furthermore, they found that BmCDA7 exhibited the highest activity, while BmCDA8 showed lower activity. BmCDA8 also showed activities toward (GlcNAc)3–6, and the kcat/Km values increased as the degree of polymerization increased. Together with the gene expression patterns, BmCDA7 and BmCDA8 may be involved in PM chitin modification at feeding stages, while BmCDA6 may play a role in PM chitin protection at the mid-molt stage. Except for the biochemical properties of insect midgut CDAs, great progress was achieved with regard to the activity of epidermis CDAs. Unlike midgut CDAs, the deacetylation activity of BmCDA1 was detectable only when the cuticular chitin-binding protein CPAP-3A1 was added.33) Taken together, the mechanism of insect CDAs to exhibit deacetylation activity was complexed and varied.
2.2. Chitin-binding activityThe affinity of insect CDA to chitin is related to the physical properties of chitin, and different CDAs show different selectivities. Research results showed that CDA in the midgut of Trichoplusia ni was tightly bound to the peritrophic membrane (PM).20) The binding of CDA to chitin could only be destroyed by the competitive chitin-binding agent, the fluorescent calcium protein. Of insect CDAs, only group V CDAs have no chitin-binding domain, but group V CDAs still have a strong chitin-binding capacity. Recent results indicated that Bombyx mori CDAs, BmCDA6, BmCDA7, and BmCDA8, all have the binding activity toward PM chitin and colloidal chitin.32) It is also worth noting that they exhibited similar binding abilities toward the same kind of chitin substrate. Considering the different enzymatic activities of the three CDAs, it seems that the chitin-binding activity was not affected by their catalytic activity.
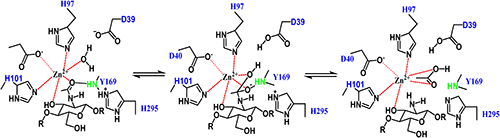
Currently, most of the polysaccharide deacetylases that belong to the carbohydrate esterase 4 (CE-4) family, such as ClCDA from Colletotrichum lindemuthianum,34) VcCDA from Vibrio cholerae,35) and peptidoglycan deacetylase PdaA from Bacillus subtilis (BsPdaA),36) have the same catalytic mechanism. The crystal structure of VcCDA, combined with its biochemical properties, suggested that the catalytic mechanism of CE-4 enzymes belongs to the generalized acid-base catalysis (Fig. 3). In the catalytic reaction process, a histidine acts as a catalytic acid, and an aspartic acid acts as a catalytic base. At the same time, a tetrahedral oxygen intermediate stabilized by zinc ions is formed. The crystal structures of insect CDAs indicate that the catalytic mechanism of insect CDAs is slightly different from that of bacteria and fungi, but some key elements in the catalytic mechanism are conserved in insects, bacteria, and fungi. Therefore, the catalytic mechanism of bacteria and fungi can provide a reference for studying the action mode of insect CDAs.
Liu et al. also studied the deacetylation mode of BmCDA8 from B. mori.33) BmCDA8 was not able to deacetylate GlcNAc or (GlcNAc)2. The deacetylation mode of BmCDA8 was investigated by analyzing the deacetylated products of (GlcNAc)3. Neither GlcN nor the monodeacetylated products of (GlcNAc)2 were present in deacetylated products, which indicates that deacetylation occurred at the first GlcNAc, which is at the nonreducing end. Thus, they deduced that BmCDA8 activity requires substrates to occupy subsites 0, +1, and +2, where 0 is the catalytic site. However, BmCDA1 requires specific accessary proteins to achieve activity (Fig. 4).

The catalytic domains of bacterial and fungal carbohydrate esterase family 4 (CE-4) enzymes are known to contain five conserved catalytic motifs (motifs 1–5) that form the active pocket for substrate binding and catalysis (Fig. 5).35,37,38) Motif 1 (TFDD) includes two aspartic acid residues; one interacts with zinc or cobalt, and the second binds the acetate released from the substrate. Motif 2 (H[S/T]xxH) contains two histidines that bind a metal ion and a serine or threonine that forms a hydrogen bond with the second histidine to stabilize the loop. Motif 3 (RxPY/F) forms one side of the active-site groove and has multiple roles, which include binding acetate, binding zinc, and coordinating the catalytic aspartate residue. Motif 4 (DxxDW/Y) forms the other side of the active-site groove, with tryptophan being the most critical residue. Motif 5 (LxH), which includes a leucine and a histidine residue, forms a hydrophobic pocket that binds the acetate methyl group and a histidine that forms a hydrogen bond with the product acetate.34,37)

Recently, alignment of the catalytic domains of CDAs from the insect midgut, fungi, and bacteria was performed.33) The results revealed that four of the five typical catalytic motifs, motifs 1 (TFDD), 2 (H[S/T] xxH), 3 (RxPY/F), and 5 (F/VxH), were conserved. However, the motif 4 (SMVDS/A) in insects is different from the conserved motif 4 (DxxDW/Y) in bacteria and fungi. Furthermore, their alignment was based on crystal structures, and the motif 4 and 5 sequences differed from those of motifs 4 (FxYD[S/A]) and 5 (Lxxxx[P/F]H) based on amino acid sequence alignment in other researches of insect CDAs.25) Besides motif conservation, a region that consisted of 55 amino acid residues between motifs 3 and 4 was found in all insect midgut CDAs but was absent from bacterial and fungal CE-4 enzymes.
4.2. Insect CDA crystal structureThe crystal structure of the first carbohydrate esterase family 4 (CE-4) CDA was reported in 2005.37) For a long time, CDA structure was only reported for fungi and bacteria, but research on insect CDA structure has recently made great progress. Liu et al. reported the crystal structure of a B. mori epidermis CDA (BmCDA1) and a midgut CDA (BmCDA8) for the first time.33) Their study found that the catalytic domain of BmCDA1 (BmCDA1-CAD) and BmCDA8 contained both a NodB homologous region conserved by members of the CE-4 family and loop insertion and special C-terminal loop regions that constitute their unique substrate-binding cleft. The NodB homology domain is an (α/β)7 barrel composed of seven parallel β-strands arranged in a barrel surrounded by six α-helices. The (α/β)7 barrel contains one loop insertion between β5 and α5. The C-terminal loops consist of α8, a pair of antiparallel β-strands, and several loops. These elements are not present in any of the other CE-4 structures determined to date. As the residue Trp/Tyr forms one wall of the active pocket, the replacement of Trp/Tyr by Ser results in an open active site for BmCDA1. The canonical Leu contributes to hydrophobic patch formation. Taken together, the active pocket of BmCDA1 is more open and wider. However, BmCDA8 contains a narrower and deeper substrate-binding cleft that passes through the active site where the catalytic reaction occurs.
The active sites of BmCDA1–CAD and BmCDA8 are both located at the top center of the (α/β)7 barrel and contain a conserved metal-binding triad across the CE-4 family, that is, the zinc ion coordinated by His–His–Asp.
In addition, through molecular dynamics simulation and site-directed mutation of protein amino acid residues, it was found that the Gln at position 125 and the Ser at position 241 are the key residues for the catalytic reactions of BmCDA8. This insect CDA structural information provides an important theoretical basis for explaining the physiological function of insect CDAs, as well as a structural basis for designing CDA inhibitors.
4.3. Comparison with fungal and bacterial CDAsTo date, six crystal structures of CDAs from fungi and bacteria have been determined: ClCDA from C. lindemuthianum,34) AnCDA from Aspergillus nidulans,39) ArCE4A from Arthrobacter species AW19M34-1,40) VcCDA from V. cholera,35) VpCDA from V. parahemeolyticus,41) and one putative CDA (EcCDA) from Encephalitozoon cuniculi.42) However, the large discrepancies in the substrate-binding site shape among these enzymes lead to varied substrate preferences and deacetylation modes.
The overall ClCDA structure is a single catalytic domain with an (α/β)7 barrel structure, which is conservative in the carbohydrate esterase family 4 (CE-4). The active site consists of five motifs and one zinc ion.34) The AnCDA active site also consists of five motifs, but the metal ion in the active site is cobalt rather than zinc.39) The VcCDA and VpCDA structures are very similar. The VcCDA carbohydrate esterase domain has an (α/β)7 topology. There are six long dynamic loops at the entrance of the VcCDA binding pocket that are used to effectively capture chitooligosaccharides in the substrate-binding pocket.35)
The similarity of the active sites indicates that insect CDAs use the same catalytic mechanism. However, the different substrate-binding cleft architectures of the CE-4 CDAs confer different catalytic properties to these enzymes. The narrow entrance of the VcCDA substrate-binding pocket is composed of six loops close to each other, which contributes a specific and high catalytic efficiency toward chitooligosaccharides.35) ArCE4A has a more open and shorter substrate-binding cleft, which confers higher binding rates for various substrates.40) Compared with VcCDA and ArCE4A, both BmCDA1 and BmCDA8 have a much longer, wider, and more open substrate-binding cleft (Fig. 6). This may explain the observed weaker activity of BmCDA1 and BmCDA8, because the lack of steric constraints within the substrate-binding clefts may reduce the effectiveness of trapping substrates once bound. The longer substrate-binding cleft formed by unique loops seems to be more suitable for insect CDAs to bind chitin fibers in vivo.33)

In recent years, great progress has been made in the research of inhibitors targeting insect chitin–related enzymes, such as chitinase.43) However, since chitinases are widely distributed in bacteria, plants, and humans, the development of specific insecticides is difficult. In contrast, CDA does not exist in humans or plants, so inhibitors targeting CDA are promising green insecticides. The development of inhibitors against this important enzyme will help to further clarify their structural features and mechanism. Additionally, successful inhibitors would be useful probes of biological function.
The AcOH formed during the deacetylation process may act as a competitive inhibitor of CDA.44) For the enzyme purified from Mucor rouxii ATC C 24905, it was shown that AcOH at a concentration of 250 mM decreased the enzyme activity to 10% of the initial value. However, the influence of AcOH on CDA from C. lindemuthianum ATC C 56676 was less significant. Optimistically, inhibitor development efforts that target carbohydrate esterase family 4 (CE-4) enzymes have had modest success (Fig. 7). Giastas et al. used Bacillus cereus peptidoglycan N-acetylglucosamine deacetylase Bc1974 as the target of anti-infective drug research and development.45) Six small molecular compounds, which include four known metal enzyme inhibitors and two amino acid hydroxamic acid compounds, were screened out to inhibit the activity of Bc1974. These small molecules can target Zn2+ in the active site of Bc1974. Using the tridentate-binding model of tetrahedral deacetylation intermediates, Benjamin et al. synthesized GlcNAc derivatives with metal-chelating groups in positions 2 and 3.46) Representative members of the CE-4 family peptidoglycan deacetylase (PgdA) from Streptococcus pneumoniae and a representative biofilm-related exopolysaccharide deacetylase (PgaB) from Escherichia coli were tested for inhibitors. Of the inhibitors evaluated, the 3-thio derivatives showed weak to moderate inhibition of PgdA. The strongest inhibitor was benzyl 2,3-dideoxy-2-thionoacetamide-3-thio-β-D-glucoside, whose inhibitory potency is dependent on the metal concentration. High-micromolar affinity inhibition of PgaB, the E. coli β-(1-6)-N-acetylglucosamine polymer (PNAG) de-N-acetylase, has been achieved using a glucosamine scaffold displaying metal chelating groups, such as an N-thioglycolyl amide.47) Monosaccharide transition-state analogues bearing a methylphosphonamidate have afforded micromolar affinity inhibition of the S. pneumoniae peptidoglycan de-N-acetylase (PgdA).48)

Based on the backbone structure of the inhibitors targeting microbial CDAs, we will explore potent inhibitors of insect CDAs via rational design and virtual screening. As the CE-4 family enzymes share the same metal-assisted general acid/base catalytic mechanism and the conserved structures of the active site, installing metal chelating functional groups that provide tighter metal coordination or mimicry of the tetrahedral intermediate on the skeleton of these inhibitors can help to find specific inhibitors of insect CDAs.
Insect CDAs play important roles in chitin metabolism. Inhibition of CDA activity can lead to developmental defects, abnormal molting, and lethality of insects, suggesting that CDA inhibitors may be developed and utilized as green pesticides. It is worth noting that insect CDAs have the same catalytic mechanism as other CE-4 enzymes. Recent advances in the structural information of insect CDAs combined with the research of CE-4 family inhibitors provided an important foundation for developing specific insecticide targeting insect CDAs.
This work was supported by the National Natural Science Foundation of China [32001938, 31772193] and the Shenzhen Science and Technology Program [Grant No. KQTD20180411143628272].