2014 Volume 31 Pages 92-110
2014 Volume 31 Pages 92-110
Metal oxide nano-microstructures are applied in photocatalytic surfaces, sensors or biomedical engineering, proving the versatile utilization of nanotechnology. However, more complex or interconnected nano-microstructures are still seldomly met in practical applications, although they are of higher interest, due to enhanced structural, electronic and piezoelectric properties, as well as several complex biomedical effects, like antiviral characteristics. Here we attempt to present an overview of the novel, facile and cost-efficient flame transport synthesis (FTS) which allows controlled growth of different nano-microstructures and their interconnected networks in a scalable process. Various morphologies of nano-microstructures synthesized by FTS and its variants are demonstrated. These nano-microstructures have shown potential applications in different fields and the most relevant are reviewed here. Fabrication, growth mechanisms and properties of such large and highly porous three-dimensional (3D) interconnected networks of metal oxides (ZnO, SnO2, Fe2O3) nano-microstructures including carbon based aerographite material using FTS approaches are discussed along with their potential applications.
Metal oxide nano-microstructures such as nanorods, nanowires, nanobelts, nanotetrapods and others are being significantly investigated due to their new and extraordinary properties appropriate for versatile nanotechnological applications1–3). Fabrication of several nanostructures from various metal oxides like ZnO, SnO2, TiO2, and Fe2O3 has already been performed using several growth techniques and their properties have been reported1–4). Among them, ZnO is one of the most investigated materials in the last decades, because of its wide and direct bandgap of ∼3.37 eV, large exciton binding energy ∼60 meV and simplicity in growth1–3, 5). Due to its hexagonal-wurtzite crystal structure, Zn and O terminated polar surfaces, different growth rates of the diverse crystal planes, quasi-1-Dimensional (Q1D) nanostructures and complex morphologies from ZnO can be synthesized. The direct bandgap which lies in the near ultraviolet -(UV) spectral region and alternating Zn and O stacking layers enable ZnO nanostructures to exhibit exceptional optical, luminescent6, 7) and electrical properties5, 8–11). Bendability is also a very important property required for different applications but bulk ceramics or metal oxides are brittle in nature1–3). However, their Q1D structures can bend elastically to larger curvatures once their thickness is in the nanoscopic range1–3, 12). Nanorods and nanowires from ZnO have already shown interesting piezoelectric properties for energy or mechanical applications1–3, 13). Illumination of ZnO nanostructures with UV light creates electron-hole pairs and thus changes their conductivity and enables its uses for photodetectors14–19). Additionally ZnO nano-microstructures are extensively investigated for light emitting diodes and gas sensing applications14, 20–22). By doping with different elements it is possible to enhance the sensing performances of ZnO nanostructures23, 24). ZnO nanostructures have also been considered as biocompatible material and therefore have been significantly employed in biomedical applications25–29). Furthermore, the interconnected nanostructures can build up microstructures or even highly porous 3D interconnected macroscopic networks which avoid risks of toxicity as a result from nanoscale particles. However, several issues like actual effect and cytotoxicity are still open25, 30). In general, various physical and chemical properties of different metal oxide nanostructures depend not only on their sizes and morphologies, but also on the synthesis techniques and experimental conditions (or technological ‘history’). Despite of such interesting applications, the scientific community is still looking for appropriate synthesis techniques, which can facilitate the versatile fabrication of different networked metal oxide nano-microstructures for practical applications in a cost-effective and scalable process.
Several synthesis methods have been utilized for growing different metal oxide nano-microstructures. Vapor-liquid-solid (VLS) mechanism31) has been a very common process for controlled growth of ZnO nanostructures like nanorods32, 33), nanowires34), nanotrees35), and interpenetrating ZnO nanosails bridge36) at junctions, etc. Other methods like hydrothermal synthesis37–39), chemical vapor deposition (CVD)40), microwave chemistry41), combustion synthesis42), plasma process43, 44), electrochemical deposition14, 45, 46), gas phase synthesis47) and flame based synthesis48–51) have been employed to grow metal oxide nanostructures, especially ZnO nanostructures. Thus, an easy scalable, cost-effective and facile synthesis method which can produce large amounts of different metal oxide nano-microstructures is still a highly demanded aspect for various applications.
Although most of the mentioned synthesis methods enable controlled growth of metal oxide nanostructures, but they lack with the feature of in-situ integration of such nanostructures directly on the chip in form of device. One requires nanoscale electrical contacts and this further hinders their application. To overcome such technological limitations a thin film fracture approach52) was invented recently. It allows direct fabrication of desired metal or metal oxide nanostructures between the electrical contacts on the sensor or device chip53–55). However, most desirable technological procedures are based on facile growth and direct integration of nanostructures on chip in a single step. Fabrication of large and porous 3D networks from interconnected metal oxide nanostructures might be an ideal choice for utilizing its nanoscale properties at macroscopic scale for different industrial technologies. It is important to mention that it has not been explored in details yet. Only few groups56, 57) have reported fabrication of 3D networks from metal oxide nanostructures using different techniques.
Recently Adelung and co-workers58, 59) have introduced a very simple and novel flame transport synthesis (FTS) approach which allows facile fabrication of versatile metal oxide nano-microstructures and their macroscopic networks. These FTS synthesized metal oxide nano-microstructures have already shown promising applications in different directions60–66). ZnO nanoseaurchin and tetrapod type structures grown by FTS approach exhibit strong potential of blocking the viral (herpes simplex virus type-1 and type-2) entry into the cells60, 61). The submicron size tetrapods can be utilized for advanced linking technologies64) and designing multifunctional composites, e.g., new concept of self-reporting material65). In this context, FTS approach offers a simple way to fabricate large size 3D networks from interconnected metal oxide nano-microstructures with desired porosity and mechanical strength. These 3D networks are stable at high temperatures (up to 〜1400°C), electrically conducting and therefore can be used in advanced technological applications57). Recently, such 3D interconnected ZnO tetrapods networks were used as templates for the guided growth of the carbon based new aerographite material, which is currently one of the least dense materials in the world67). In this paper, we present the growth of different metal oxide nano-microstructures and their interconnected 3D networks by FTS approach and its variants. Different morphologies, growth mechanisms and properties of nanomaterials are presented and discussed in detail.
Flame transport synthesis (FTS) method and its variants59) mainly require microscale diameter spherical metal particles as precursor materials, sacrificial polymer (e.g., polyvinyl butyral (PVB), etc.) and ethanol for mixing homogeneously in slurry form. In our works59), different metal particles powders (Zn, Fe, Sn, Bi, Al, Si etc.) with diameters in the range from 3 μm to 45 μm have been used (purchased from GoodFellow UK, purities 99.9% to 99.99%). PVB powder (supplied by Kuraray Europe GmbH, Germany) was used as sacrificial polymer for the presented experiments. A simple muffle type furnace which can rapidly heat up to high temperatures was used for our experiments. Also, in these experiments, no gas control or vacuum equipment is necessary because it works in normal air environment. Some ceramic plates, crucibles, and cylinders and conventional burner type equipments are required for different variants of FTS approach59).
2.2 Flame transport approach and its variantsThe first variant of FTS approach (Fig. 1a) enables the fabrication of nanoseaurchin type structures59). For this process the slurry mixture of precursor metal -microparticles, PVB powder and ethanol is prepared in appropriate ratio and coated on desired substrates (Si, Aluminum etc.). After drying the coated layer on the substrate, it is heated at different temperatures (depending upon the type of precursor metal microparticles) and formation of nanoseaurchin type structures occurs. For example, to synthesize ZnO nanoseaurchins, a temperature above 550°C is sufficient. According to our experimental evidence for iron oxide nanoseaurchins, the optimum temperature is about 900°C. By increasing the precursor metal particle ratio versus PVB in the slurry and by increasing the thickness of the coated layer, it was possible to fabricate a 3D network from nanoseaurchins (Fig. 2a) in a similar manner. The second variant of FTS approach allows direct transformation of precursor metal microparticles into metal oxide nano-microstructures in powder form (Fig. 3a–3d). The mixture Zn:PVB with ratio 1:2 was filled in the ceramic crucible and heated up to 900°C, which results in formation of different ZnO nano-microstructures in form of powder. These nano-microstructures can be harvested from the furnace and accordingly utilized for desired applications59). The yield per experiment mainly depends on the several factors like ratio of Zn to PVB, amount of Zn and PVB mixture and temperature of the furnace etc. In each standard experiment for ZnO tetrapods production (Zn:PVB = 1:2, 900°C, 30 minutes) the yield was around 30–40% and in each experiment and we harvest about 15–20 grams of ZnO tetrapods depending upon initial conditions. The third variant of FTS approach utilizes an in-house ceramic cylinder arrangement (Fig. 5a) and it enables controlled synthesis of different quasi-1D nano-microstructures and their networked bridges. The fourth variant utilizes a simple burner approach (Fig. 6a) in which the precursor metal microparticles are directly inserted into the oxygen and propane gas mixture inside the burner tube. On their way in the flame, these precursor metal microparticles are converted into various metal oxide nano-microstructures within milliseconds and can be collected in form of powder or in-situ integrated on the substrate/chip as per requirements59). The main aim of this paper is to provide an overview of different structures, which can be synthesized using proposed FTS approaches for practical applications. More technical details about ratios, growth conditions, temperature time and substrate positions can be found in previous works58, 59).
2.3 Characterizations:The obtained nano-microstructures by FTS approach were characterized using scanning electron microscope (20 keV Philips-FEI XL30 equipped with LaB6 filament). Electromechanical measurements of Q1D, 2D and 3D networks were performed by using a home made setup which includes a micro-manipulator, a Keithley 2400 Sourcemeter, and a computer-controlled laboratory balance with the help of a LabView program59).
Fig. 1 demonstrates the growth mechanism of nanoseaurchin type structure and few examples of already synthesized nanoseaurchins from different metal oxides (ZnO, SnO2, Fe2O3, Al2O3, Bi2O5). To grow ZnO nanoseaurchins, a dilute slurry mixtures from Zn, PVB and ethanol [Zn: {PVB:Ethanol}=1:3{1:2}] was prepared and coated on Si substrates. After drying the slurry coated substrate was heated at 600°C in the muffle furnace for 2 hours. Depending upon the requirements the ratios of Zn, PVB and ethanol were varied and the coated substrates were heated above 600°C for different durations to synthesize various types of seaurchins59). In similar manner, nanoseaurchins of several other metal oxides have been successfully grown and can be found in previous work59).
For the growth of core-spike nanoseaurchins (Fig. 1), the crystalline quality of precursor metal micro-particles as well as processing temperature play very important roles. The sacrificial polymer (PVB) and ethanol mixture exhibit double roles: (i) it maintain necessary separation between precursor microparticles which prohibits the agglomeration of metal microparticles and (ii) during heating it provides necessary local environmental control for nanospike growth on the surface of microscopic core. Also above certain temperature (below the nanospike growth) the PVB decomposes entirely and leaves no fingerprints behind in the grown seaurchins or nanostructures, since it contain only C and O. The heating ramp rate of the furnace is the most important parameter for growth of nanoseaurchins, e.g., faster heating rates result in a better growth of such micro-nanostructures (we used furnace with ramp rate above 100°C/min). It has been observed that the metal oxide nanospikes were synthesized on the surface of precursor metal microparticles only when the growth condition is far from thermal equilibrium. Under such circumstances, the kinetic growth processes dominate and it leads to a nanospike growth on the substrate/crucible surface. This type of the spherical precursor microparticles exhibit surface defects, like reconstructed surface and grain boundaries68). It seems that the grain boundaries most likely offer the energetically favorable nucleation sites to initiate the nanospike growth68). These nanospikes were investigated in details using cross-sectional high resolution transmission electron microscopy along with precession electron diffraction studies and it was observed that they consist of twin boundary propagating along the c-axis69). Such ZnO nanoseaurchin structures show their promising application as potential candidate for blocking the viral (HSV-1) entry into the cells60). Further work on this kind of micro-nanostructures is in progress.
As mentioned already that growth of 3D interconnected network from metal oxide nanostructures is a highly demanded aspect for practical applications. Using the growth concept of nanoseaurchins, we have successfully grown large 3D interconnected networks from core-spike nanoseaurchins. The schematic drawing for 3D network growth is demonstrated in Fig. 2a in which a thick layer of slurry mixture is coated on the substrate. The ratio of metal microparticles, PVB and ethanol is kept in such a manner that the precursor metal particles are homogenously dispersed in the slurry and during coating they are well separated by sacrificial polymer layer (mixed with ethanol). After drying the substrate with slurry is heated rapidly up to higher temperature (typically ∼600°C for ZnO, and ∼900°C for Fe2O3, SnO2, Bi2O3 and Al2O3)59). Because of rapid heating process, the growth of nanospikes on surface of precursor metal particles is initiated like demonstrated in Fig. 1. The sacrificial polymer layer plays a crucial role here as it maintains the necessary separation between microscopic particles and at the same time nanospikes grow and form interconnecting bridges between individual cores59). These interconnecting nanospike bridges provide mechanical strength to the network59). Using this concept, large 3D interconnected networks from ZnO nanoseaurchins were successfully grown and are shown by zoom-in series of SEM images in Fig. 2 (b–d) (from left to right). The interconnecting nanospike bridges can be clearly seen in high magnification SEM image in Fig. 2d.
A large 3D network from ZnO nanoseaurchins grown on Aluminum substrate (shown in Fig. 2e) following a similar FTS approach. Such kind of networks were investigated by SEM and confirmed that it is entirely made from interconnecting core-spike nanoseaurchins, as presented in Fig. 2(f). Its corresponding higher magnification SEM image is shown in Fig. 2(g). The 3D networks from ZnO nanoseaurchins have been successfully grown and optimized. However experiments for other metal oxide 3D networks are currently under progress.
The second variant of FTS approach enables direct conversion of precursor metal microparticles into nanostructures within the flame created due to burning of sacrificial polymer (PVB). The mixture of precursor metal microparticles and PVB (1:2 ratio by weight) is filled in the ceramic crucible which is placed inside the furnace and heated rapidly up to temperature of 900°C (for Zn microparticles). Because of high heating rate the PVB starts burning and creates intense flame59). Then, the precursor metal microparticles travel in the flame stream and due to of high flame temperature are converted in ZnO nano-microstructures. After reaching 900°C, the temperature of the furnace is maintained constant for different time spans (ranging from 30 minutes to 4 hours depending upon the requirements) for proper growth of specific nano-microstructures. The role of sacrificial polymer (PVB) is very important in FTS process, as it maintains the separation of metal microspheres, creates the flame and also enables necessary environmental conditions for nano-microstructures growth59).
The conversion principle of precursor metal microparticles into the flame is demonstrated in Fig. 3a. For simplicity reasons only two types of structures (tetrapods and multipods) are shown in the schematic however structures with various complex morphologies have been grown. Fig. 3b shows the digital camera image of the on going FTS process and the intense yellow flame in the centre shows the turbulent stream of flame in which microparticles are being converted into nano-microstructures. Once the process time is achieved, the furnace was turned off. After cooling one can see the crucibles and the base of the furnace are entirely covered with snowflake type white powder (Fig. 3c). These snowflake type powders consist of tetrapods, multipodes, branched ZnO nano-microstructures depending upon the location in the furnace. The snowflake type powder was harvested from positions 1 and 2 (Fig. 3c) was analyzed inside SEM. Morphological studies of the powder harvested from position 1 (Fig. 3c), i.e., from the crucible, revealed that it mainly consists of ZnO tetrapod structures with different sizes as shown by SEM images in Fig. 3(d–f) at increasing magnifications (from left to right). However, the fluffy powder at the base (position 2, Fig. 3c) consists of ZnO tetrapods, ZnO multipodes and several other complicated shapes as evident by SEM images in Fig. 3(g–i) at increasing magnifications (from left to right). The ZnO tetrapod and multipode structures can be harvested and can be utilized accordingly for desired applications. The ZnO tetrapods have shown promising applications, as antiviral agents60, 61), in advanced linking technology64) and designing self-reporting composites65).
Since growth of desired 3D networks was the strong motivation for our research, the harvested ZnO tetrapods from second variant of FTS were further processed to synthesize different interconnected networks59). Appropriate amounts of tetrapods were filled in open cylindrical ceramic scaffolds, pressed and reheated at high temperature (∼1150°C) for 4 hours. Such a reheating results in the formation of interconnections between tetrapod arms providing mechanical strength to the entire network59). For example, the 3D ZnO networks fabricated using this approach is shown in Fig. 4a. These 3D networks were analyzed inside SEM in details to study the underlying growth process. Fig. 4(b–d) shows the SEM images taken inside the network at different magnifications. It is clearly visible that at some places the tetrapod arms interpenetrate fully (Fig. 4d is a high magnification of Fig. 4b) and at some places they interpenetrate partially (Fig. 4c). It seems that formation of interconnections initiates from the positions where the arms are touching each other during re-heating. However, further investigations for understanding the exact mechanism of arms’s interpenetrations are under progress59).
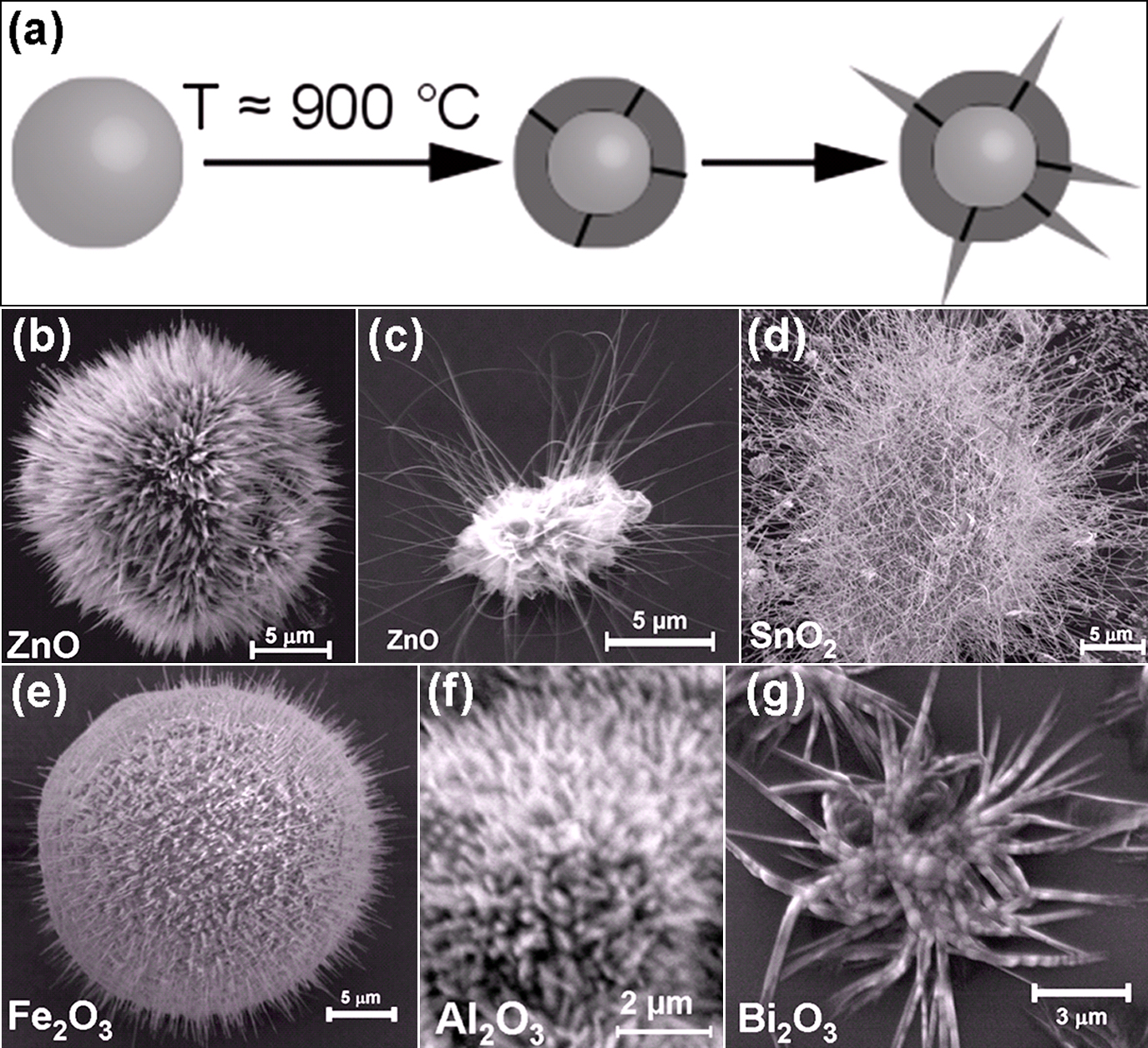
(a) Schematic drawing demonstrating the formation of core-spike nanoseaurchin type structure. (b)–(g) Nanoseaurchins of different metal-oxides (ZnO, SnO2, Fe2O3, Al2O3, Bi2O3) grown by using FTS approach. (b) & (c) Two typical examples of ZnO nanoseaurchins grown at 900°C. (d) Tin oxide nanoseaurchin structure grown at 950°C. (e) Core-spike nanoseaurchin from iron-oxide obtained at 900°C. (f) Aluminum oxide nanoseaurchin grown by FTS approach (shown in 1a). (g) Bismuth oxide nanooctopus type structure with branched nanoscopic arms fabricated at 900°C. [Partially reproduced from Fig. 1 with permission from59)].
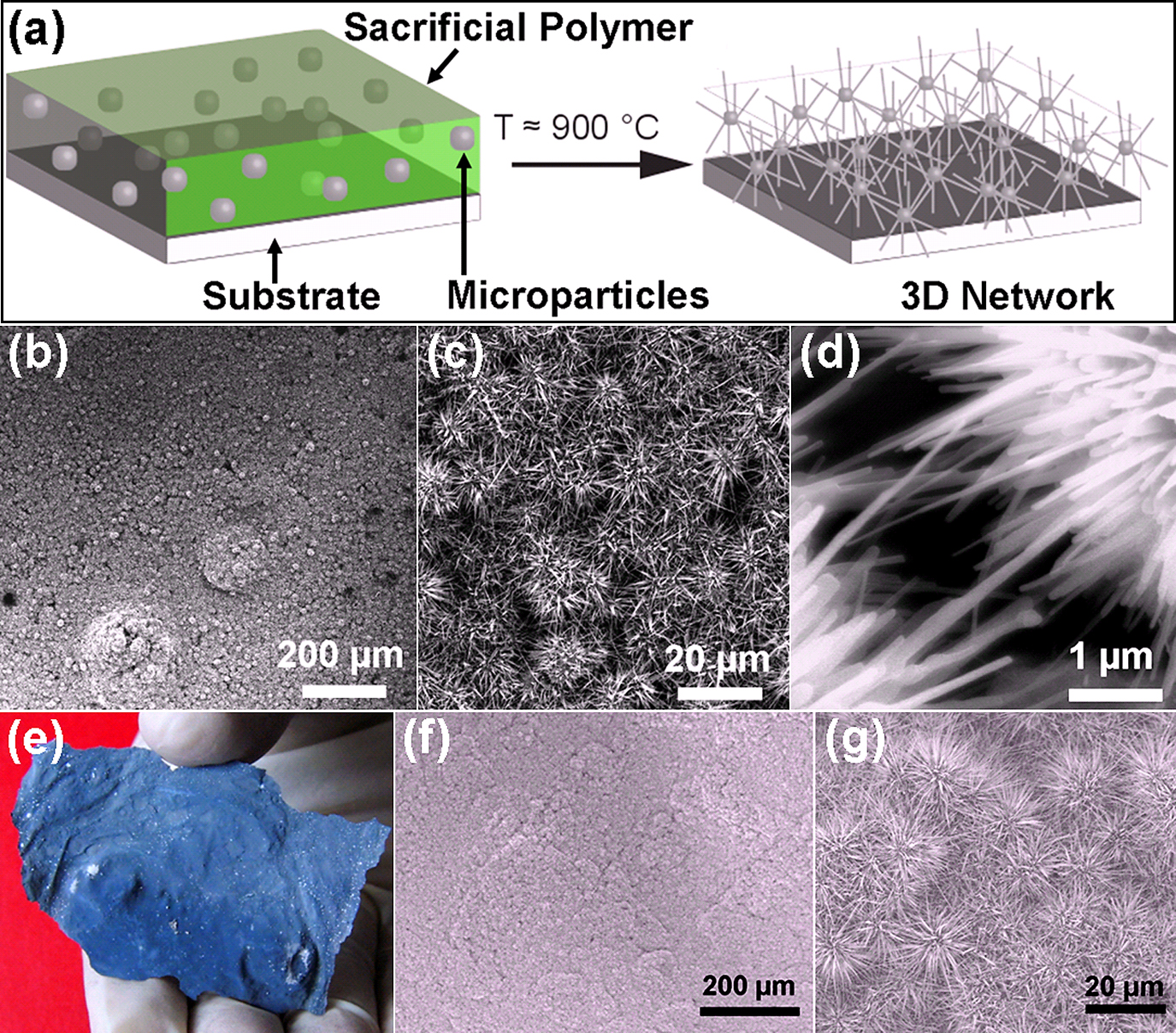
(a) Fabrication principle of 3D interconnected networks by assembling nanoscale seaurchins. The precursor metal microparticles are mixed with sacrificial polymer and ethanol and then coated on substrate. During heating, the sacrificial polymer decomposes completely and at the same time the nanospikes grow and establish the interconnections among microscopic cores which provide the mechanical strength to the network. (b)–(d) SEM images from a 3D interconnected ZnO nanoseaurchins network at increasing magnifications (from left to right) synthesized by approach described in (a). (e) Digital camera image a 3D ZnO nanoseaurchins network synthesized using Aluminum substrate. (f) and (g) SEM images from the 3D interconnected network (shown in e) at higher magnifications (left to right). [Partially reproduced from Fig. 1 with permission from59)].
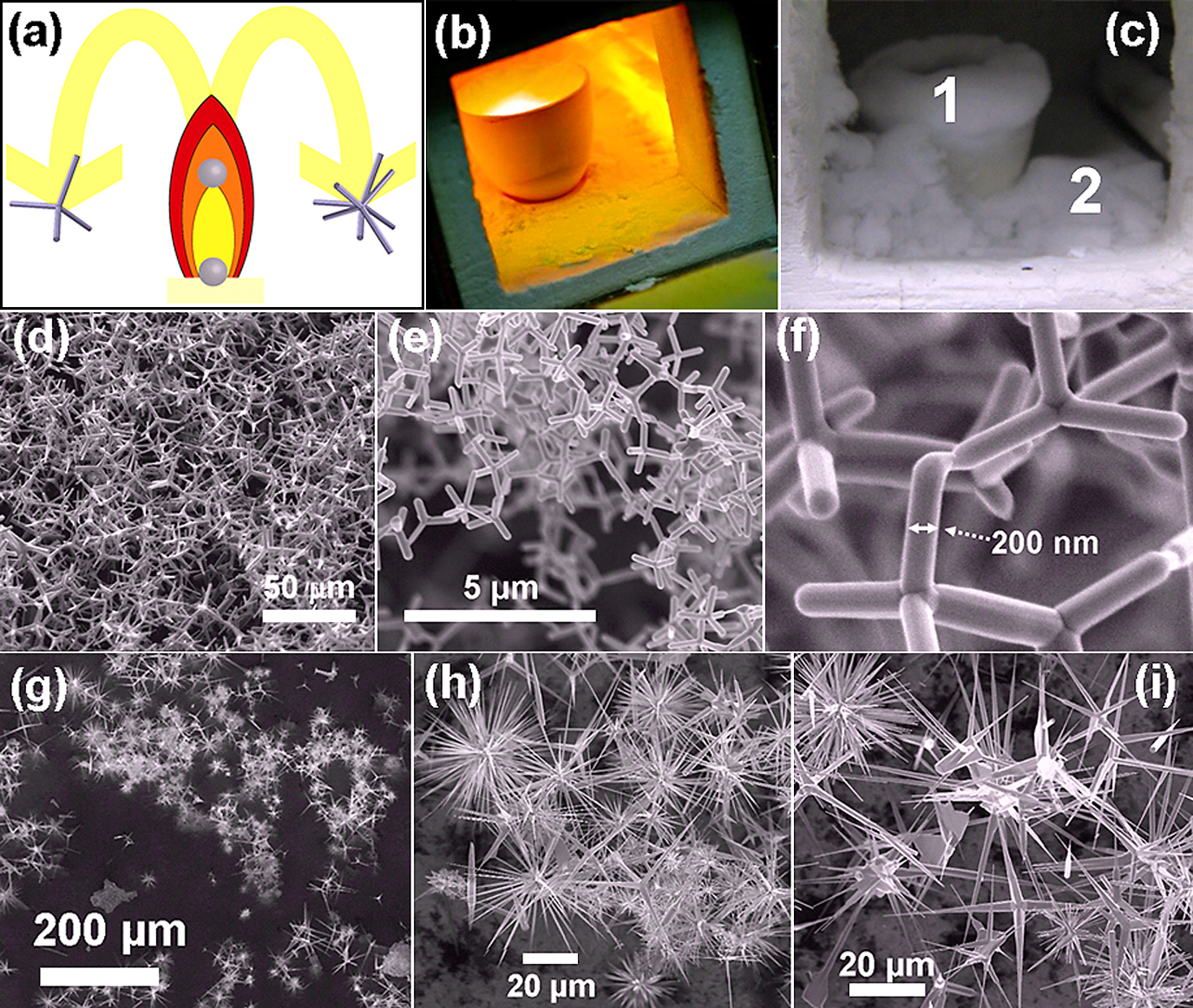
Flame transport synthesis of different nano-microstructures: (a) Schematic drawing showing the conversion principle of precursor metal microparticles into different nanostructures by flame; (b) digital photograph of ongoing flame transport process in the furnace. The mixture of precursor metal microparticles and sacrificial polymer powder are filled (∼60% by volume) into ceramic crucible and then heated in the furnace at 900°C. The intense yellow flame in the centre of crucible shows the ongoing flame transport process. (c) Digital photograph of the furnace chamber after the flame transport process. The converted nano-microstructures are deposited everywhere inside the furnace. These structures can be harvested from different locations (e.g., 1 and 2 within the crucibles and at its base). (d)–(f) SEM images of ZnO tetrapods at increasing magnifications (from left to right) harvested from location 1. (g)–(i) Zoom series (from left to right) of SEM images from the fluffy powder harvested from the base of the furnace (location 2 in image c) showing formation of tetrapod, multipods and their mixed structures. [Partially reproduced from permission from59)].
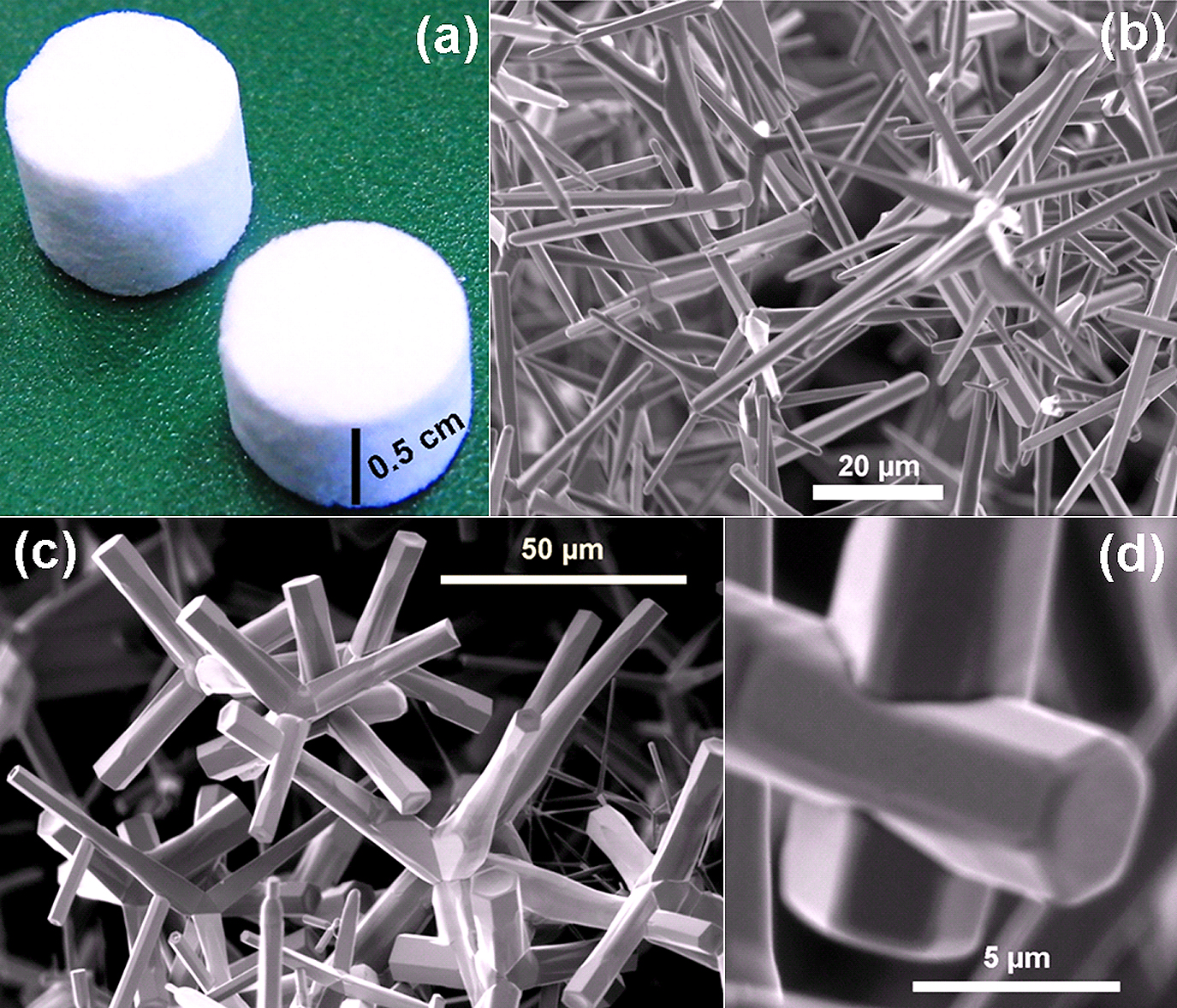
Fabrication of the 3D interconnected networks from the ZnO tetrapod powder (shown in Fig. 3d). Appropriate amounts of ZnO tetrapods were filled in ceramic crucible and re-heated at high temperatures (between 1100°C to 1200°C) for 4–5 hours. (a) Digital camera image of the 3D network tablets obtained after heating the ZnO tetrapods in the ceramic scaffolds (at 1150°C for 4 hours). (b)–(d) SEM images from these 3D network tablet confirming the formation of interconnections between the tetrapod arms. Formation of partial (c) and full (d) interpenetrations between the tetrapod arms can be seen. [Partially reproduced from permission from59)].
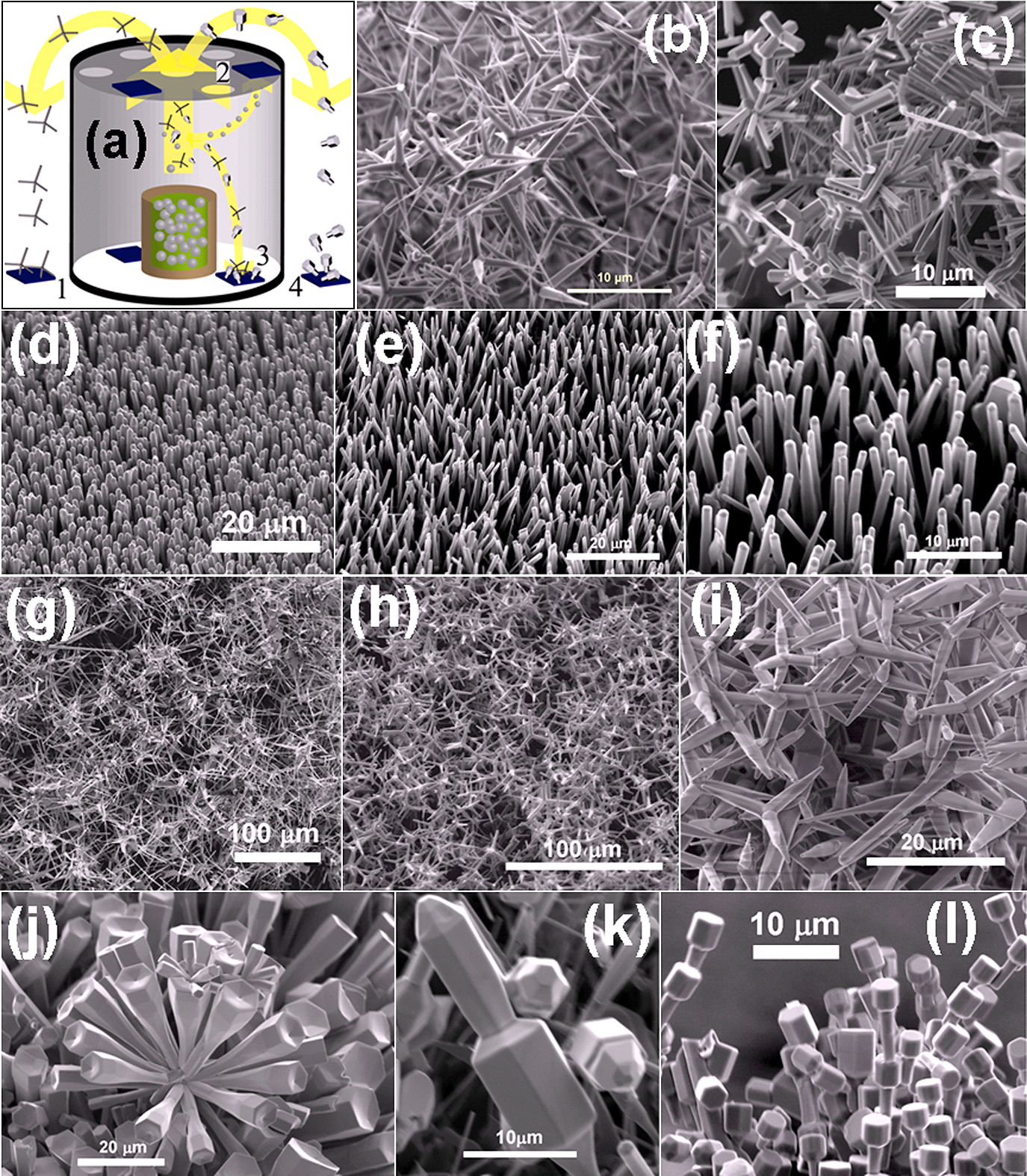
(a) Schematic drawing demonstrating the controlled variant of FTS approach using a ceramic cylinder. The crucible in centre contains the slurry mixture and the substrates are mounted at positions 1 to 4. (b) & (c) SEM images for different ZnO tetrapod structures grown at substrate mounted at position 1. At position 2 (top cover of cylinder having holes) the substrates are symmetrically mounted with the desired surface pointing vertically downwards towards the crucible. Typically quasi-1D nano-microstructures and their arrays grow at position 2. (d)–(f) SEM images of different ZnO nanorods array grown on Si substrate at position 2. (g)–(i) SEM images of different types ZnO tetrapods networks grown at position 3 inside the cylinder. (j)–(l) SEM images of different types of ZnO structures i.e., flowers, dumbbells, bolts and other morphologies were grown at substrate mounted at position 4. Nano-microstructure grown at positions 1 and 4 are almost same. For demonstration reasons, only few structures are shown here. [Partially reproduced from Fig. 2 with permission from59)].
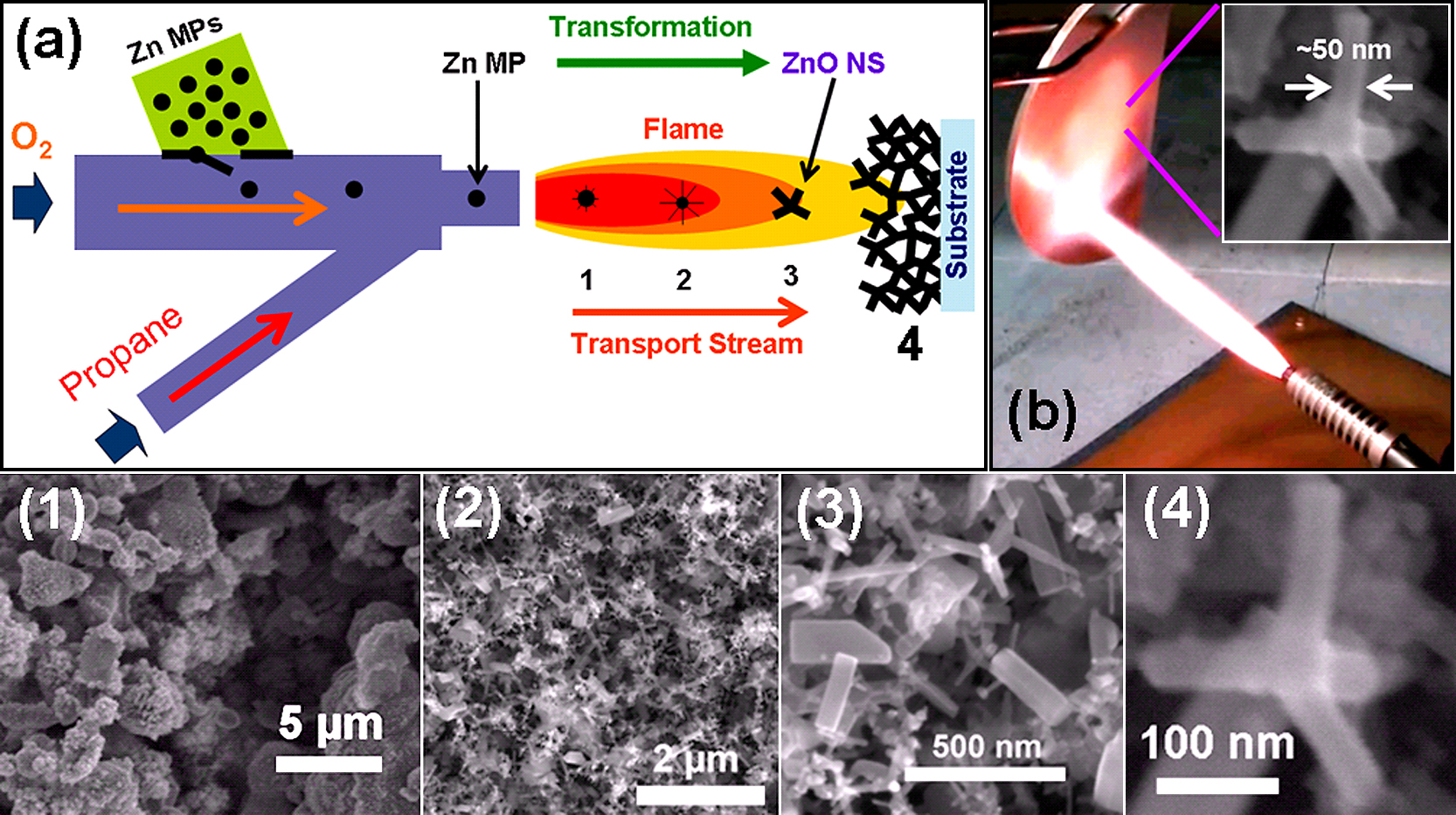
(a) Modified FTS technology using burner approach. The precursor metal microparticles are inserted into the burner, which are transported and converted to metal oxide nano-microstructures in the flame. The transformation of precursor microparticles depends on their time of travel in the flame which are marked 1–3 and at 4 corresponds to substrate where converted structures are collected (for simplicity only tetrapod shape is shown but typically various shapes can be obtained). (b) Digital camera image of a copper plate coated with ZnO tetrapods. Inset in (b) shows the SEM image of nanoscale ZnO tetrapod deposited on the Cu plate. SEM images (1–4) shows the transformation of precursor ZnO microparticles into ZnO nanostructures. [Partially reproduced from Fig. 4 with permission from59)].
Based on proposed approach macroscopic and highly porous (up to 98%) 3D interconnected networks were fabricated using ZnO tetrapods59). These networks are electrically conducting and high temperatures stable (up to 1400°C). Flexibility of 3D porous networks is a very important property which enables their use for various applications. It was observed that by varying the density, one can tune the elastic modulus of these networks from few kPa to rubber elastic region (less than 100 MPa) and thus they fall in the category of flexible ceramics59). These flexible conducting highly porous 3D interconnected networks exhibit potential applications for new advanced technologies. Recently, these 3D networks have been used as templates for guided growth of carbon based aerographite material67).
The third variant of FTS offers controlled growth of different Q1D metal oxide nano-microstructures employing the cylindrical arrangement as shown in Fig. 5(a). The precursor metal microparticles, sacrificial polymer and ethanol mixture (in form of slurry) is placed in the crucible and the substrates can be mounted at positions 1 to 4 (Fig. 5a) for different nano-microstructures. The cylindrical arrangement is inserted into the furnace and heated at high temperatures in air environment. The cylinder arrangement enables further control on the nanostructure growth process and Q1D nanostructures, like nanorods, nanowires, nanonails and their large arrays can be grown in a controlled manner59). The conversion mechanism of precursor microparticles in the flame is similar like mentioned in Fig. 3(a) but inclusion of ceramic cylinder adds extra features. At positions 1 and 4 (Fig. 5a), the nanostructure growth process is almost similar to that demonstrated in Fig. 3.
Nano-microstructures grown at position 2 and 3 (Fig. 5a) are quite different, because position 3 is located inside the cylinder at the base surrounding the crucible and position 2 on the top holes, where substrates are mounted vertically downwards. SEM studies from structures deposited on position 1 (Fig. 5a) confirmed the growth of different types of ZnO tetrapods, as shown in Fig. 5b and Fig. 5c. Growth of different Q1D nanorods and their arrays occurs on substrates mounted at position 2, (Fig. 5a). On substrates at position 3, located inside the cylinder, more converted nano-microstructures are deposited during growth process and therefore 3D networks are formed. These 3D networks are quite different as compared to those obtained by reheating the tetrapods (Fig. 4). Since growth and interpenetration simultaneously occur at position 3, these 3D networks are much stiffer and exhibit relatively higher elastic modulus59).
The fourth variant of FTS approach utilizes a burner equipped with Oxygen and Propane gases for the flame as shown in Fig. 6a59). The precursor Zn microparticles are inserted into the gas stream in controlled manner and they travel along with the flame when coming out from the nozzle. During their travel, these precursor metal microparticles are converted into nano-microstructures because of very high flame temperature of the flame. These nano-microstructures can be deposited on the substrates or collected in form of powder depending upon the requirement.
The 3D interconnected networks can be synthesized if process is continued for longer durations at same locations59). The conversion of Zn microparticles into nano-microstructures depends on transport time within the flame and transformation stages as marked by positions 1, 2 and 3 (Fig. 5a). A large copper plate coated with ZnO tetrapods using burner approach is shown in Fig. 6b. The SEM images (1–3) presented in Fig. 6 correspond to particle transformation stages shown in Fig. 6a. The image 4 in Fig. 6 shows SEM of a single ZnO nanotetrapod, which was coated on copper plate. More details about different experiments are elaborated in previously published paper59) and experiments are still under progress to optimize the growth of other metal oxide nano-microstructures. The FTS grown nano-microstructures have already shown promising applications and few are summarized in the next section. Recently using burner-FTS approach, an interconnected ZnO nanotetrapods network bridge was directly integrated between electrodes in a chip for UV photodetector applications which demonstrated very fast photodetection response70).
The synthesized structures by FTS approach were tested for antiviral applications and it was observed that ZnO nanoseaurchins and tetrapods (shown in Fig. 7a and 7b) block the HSV-1 viral entry into cells60). The confocal microscopy images (A–C) in Fig. 7c confirm the attachment of viruses on the surface of ZnO structures. The plot D in Fig. 7c shows the HSV-1 entry level into the cells in presence of unilluminated and illuminated ZnO structures with UV light. It was observed that in presence of such ZnO structures, the HSV viral entry level goes down as some viruses get attached on the ZnO surface. After UV illumination of these ZnO structures, the HSV-1 viral entry into the cells is significantly decreased because of enhanced attachment of viruses on the ZnO surface60).

Antiviral activity of the ZnO nanoseaurchins and tetrapods against herpes simplex virus type -1 (HSV-1). The SEM images (a) & (b) show morphology of the ZnO nano-microstructures used for HSV-1 experiments. (c) Confocal microscopy results (A–C) and antiviral activity (D) of ZnO structures: Image (A) shows the confocal microscopy image of ZnO tetrapods. The confocal microscopy images in (B) and (C) shows the attachment of HSV-1 with ZnO structures (viruses were leveled with green markers). Plot in (D) demonstrates the antiviral activity of ZnO nano-microstructures without and with UV illumination. (d) Schematic model demonstrating the blocking of HSV-1 entry into cells by ZnO structures. When illuminated with UV light, these ZnO structures significantly bind HSV-1. [Reproduced with permission from60)].
The HSV-1 entry mechanism into the cells is demonstrated by schematic drawing shown in Fig. 7d. The HSV-1 virus exhibits heparan sulfate (HS) groups as virion envelop which interacts with gD-receptors on the cells and enter into the cells. The presence of ZnO nano-microstructures screens the HSV-1 entry into the cells as some viruses are attracted by the oxygen deficient surfaces of ZnO nano-microstructures. After preparation, the ZnO nano-microstructures are several times under sun-shine, it is sufficient to create oxygen vacancies60). After UV illumination, further O vacancies are created in such ZnO structures which enhance the virus attachments on the ZnO surface and results in the significantly decreased HSV-1viral entry into the cells decreased. More details can be found in previous work60).
4.2 Antiviral activity of nano-micro ZnO tetrapods against HSV-2Since herpes simplex virus type 2 (HSV-2) is more fatal as compared to HSV-1 and antiviral community is still waiting for appropriate treatments. We utilized the ZnO tetrapods to study the HSV-2 viral entry into the cells61) and its antiviral activity is demonstrated in Fig. 8. The SEM images of ZnO tetrapods used for experiments with HSV-2 are shown in Fig. 8(a–c) at increasing magnifications (from top to bottom). The confocal microscopy results of ZnO tetrapods without and with HSV-2 viruses are shown in Fig. 8(d) and Fig. 8(e, f), respectively.
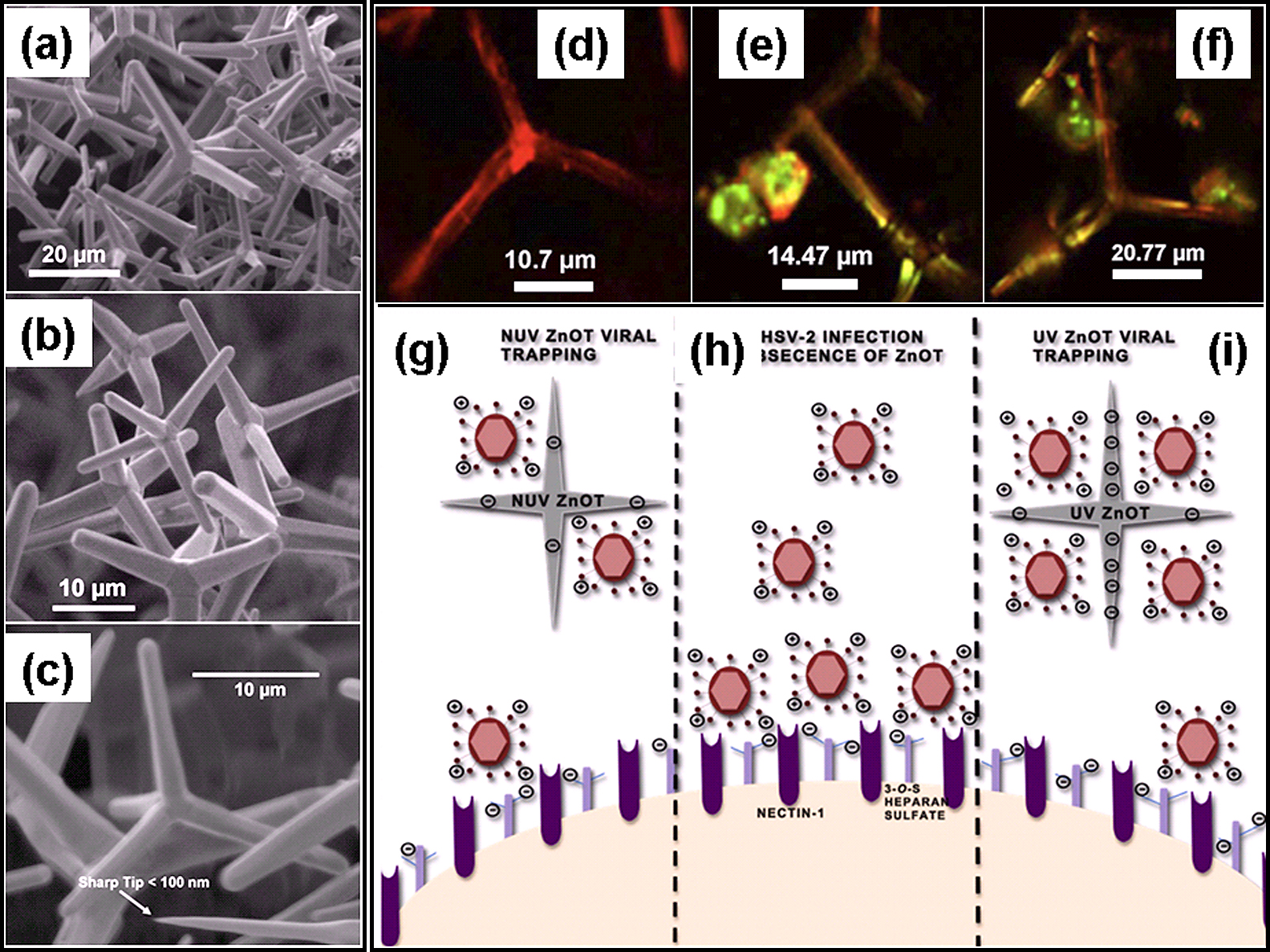
Antiviral activity of ZnO tetrapods against herpes simplex virus type -2 (HSV-2). (a)–(c) SEM images of ZnO tetrapods utilized for HSV-2 experiments. (d)–(f) Confocal microscopy images of ZnO tetrapod (d) without and (e, f) with HSV-2. The viruses are leveled with green markers and confocal microscopy images in (e) and (f) show that they are attached with ZnO tetrapods. (g)–(i) Schematic drawings show the role of ZnO tetrapods in HSV-2 entry into the cells. (h) In absence of ZnO tetrapods viruses enter into the cells in normal manner. (g) In presence of ZnO tetrapods some viruses are attached with ZnO tetrapods and thus their entry into cell decrease. When these ZnO tetrapods are illuminated with UV light, more viruses are attached with tetrapods and this further reduces the viral entry into cells. [Reproduced with permission from61)].
The HSV-2 viruses were labeled with green markers using a dye. The viral entry mechanism of HSV-2 viruses into the cells in presence of ZnO tetrapods is demonstrated in Fig. 8(g–i). As mentioned above, in presence of ZnO tetrapods, some viruses are attached at the surface of ZnO tetrapod arms due to inherent oxygen vacancies (Fig. 8g). In absence of ZnO tetrapods, the HSV-2 viruses make normal entry into the cells in natural manner (Fig. 8h). After UV illumination on such ZnO tetrapods, the HSV-2 viral entry into the cells goes significantly down (Fig. 8i) because more viruses are attached on the surface of ZnO tetrapods arms due to additional oxygen vacancies created by UV illumination61). Few studies relating to blocking capability of these ZnO tetrapods have already reported in recently published articles60, 61, 63). Further in-vivo experiments are under progress to understand the process in more details. Similar phenomenon was observed in case of SnO2 nanowire networks, synthesized by FTS approach shows strong potential of blocking the HSV-1 entry into the cells62). For biomedical applications of different nano-microstructures, cytotoxicity is very important issue and these nano-micro scale ZnO tetrapods exhibit very low level of cytotoxicity as compared to conventional spherical nanoparticles and ZnCl2 reference71), which implies towards better utilization in biomedical engineering.
4.3 ZnO nano-micro tetrapods for advanced linking technologyA brand new method of joining polymers with low surface energy was developed by the utilization of the ZnO nano-micro tetrapods (Fig. 9) produced by the second FTS variant shown before59). This method employs the convex shape of tetrapodal microparticles which works as an anchor to mechanically interlock two polymer layers together. Its efficiency was demonstrated by joining two extremely difficult-to-join polymer layers, namely the poly(tetrafluorethylene) (PTFE) and cross-linked poly(dimethylsiloxane) (PDMS)64). Both polymers have very low surface energies and it was difficult to find a single report describing that both polymers can be strongly joined by any technique. Even PTFE alone is notoriously difficult to modify. The surface modification methods developed before, such as plasma treatment and ion beam treatment, require specific instruments. Chemical treatments are difficult and involve hazardous chemicals with PTFE, which is highly chemically inert. The new method achieved a peeling strength of 220 N/m by an easy applicable approach, without modification of the chemical composition of each polymer layers64).
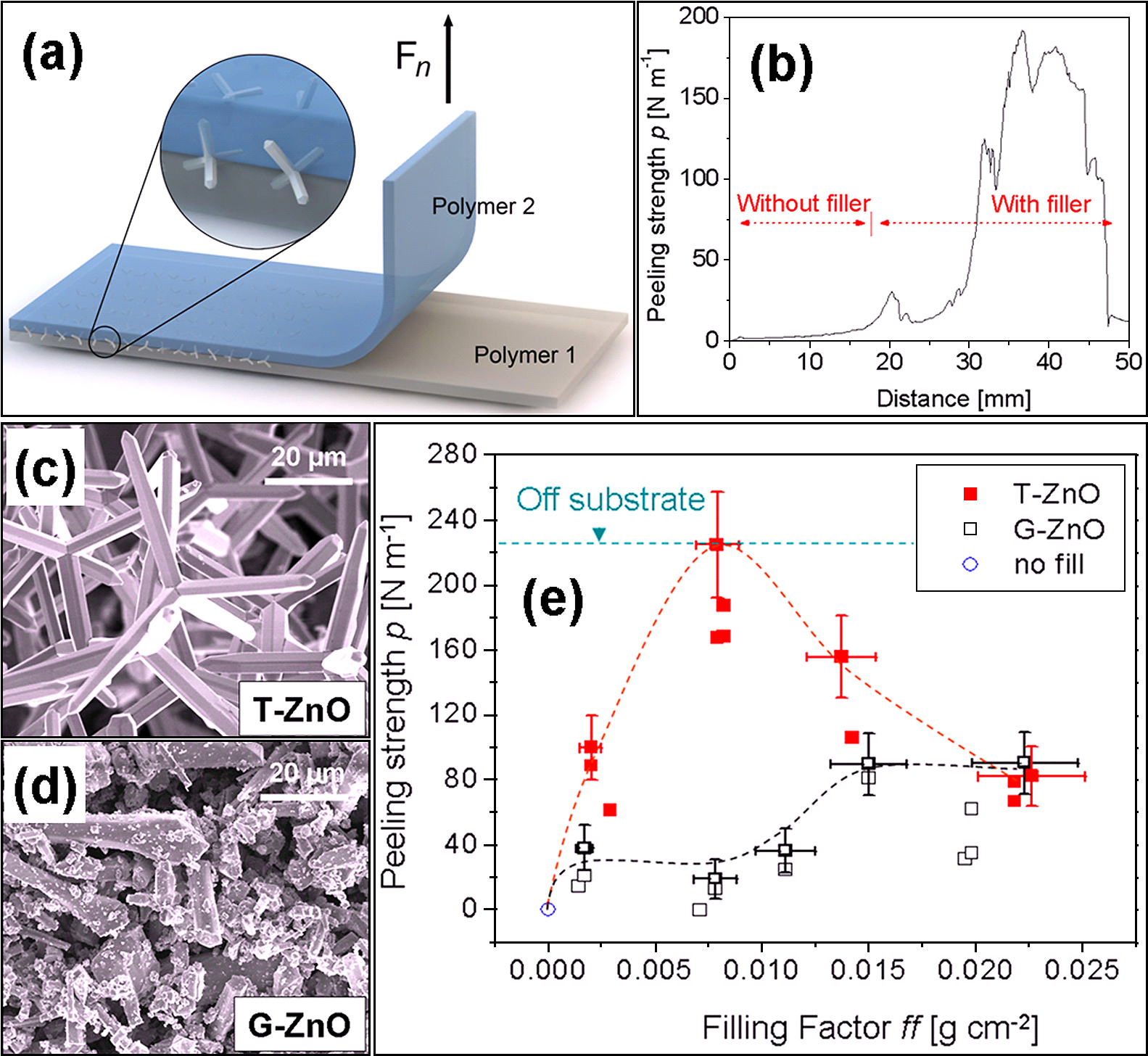
ZnO tetrapods used as cross-linkers for joining the least surface energy polymers (PDMS and PTFE). (a) Schematic drawing demonstrating the experiment for testing the adhesion strength between two polymer layers with tetrapods at the interface as cross-linkers. (b) Peeling strength between PTFE and PDMS polymer layers without and with ZnO tetrapods. (c) & (d) SEM images of ZnO tetrapods (T-ZnO) and ground ZnO tetrapods (G-ZnO). (e) Peeling strength between PDMS and PTFE layers when filled with T-ZnO and G-ZnO as fillers. Utilizing ZnO tetrapods as cross-linkers, the peeling strength between PDMS and PTFE layers significantly increased. [Reproduced with permission from64)].
Fig. 9a demonstrates the adhesion technology between two polymer layers employing ZnO tetrapods (T-ZnO) at the interface between two layers. It was observed that with the T-ZnO at the interface the peeking strength was improved significantly (Fig. 9b). In order to compare the effect of shape, some ZnO tetrapods (Fig. 9c) were ground (G-ZnO), which resulted in microrod type structures (Fig. 9d). These G-ZnO (ground ZnO tetrapods) structures were also embedded at the interface between two polymer layers and peeling strengths from both (T-ZnO and G-ZnO) were compared which are shown in Fig. 9e. It can be clearly seen that employing T-ZnO gives much better adhesion as compared to G-ZnO. Since there was no chemical treatment applied to ZnO structures and polymer surfaces, the strong adhesion is purely due to mechanical interlocking. Therefore the concave shaped particles such as tetrapods produced by FTS approach has shown unique potential in improving the mechanical adhesion of polymer laminates64). More details can be found in report by Jin et al.64)
4.4 ZnO nano-micro tetrapods as fillers for fabricating self-reporting compositesSemiconducting tetrapod type structures have already shown interesting stress dependent luminescent responses72, 73). An important possible application of tetrapodal shaped ZnO nano-microstructures in polymers is the development of a new concept for self-reporting materials65). The concept assumed that, the deformation of the crystals induces changes in the photoluminescence (PL) signal of the ZnO nano-microtetrapods and therefore it can be used to indicate the stress state of the material. This concept was tested by embedding the tetrapodal particles in an elastomeric matrix as shown in Fig. 10a. Typical SEM image of ZnO tetrapods used in the experiments are shown in Fig. 10b and digital camera image of the final prepared T-ZnO: PDMS (Polydimethylsiloxane) composite is shown as inset in Fig 10b. Desired size and shape of composite can be fabricated as reported by Jin et al.65) The PL spectra of T-ZnO: PDMS composites with respect to tensile stress are shown in Fig. 10c. It can be seen that the intensity of blue PL peak (from UV excitons in ZnO) remains constant but intensity of green PL peak (∼523 nm) has increased which most likely appears from surface defects in ZnO10, 11).
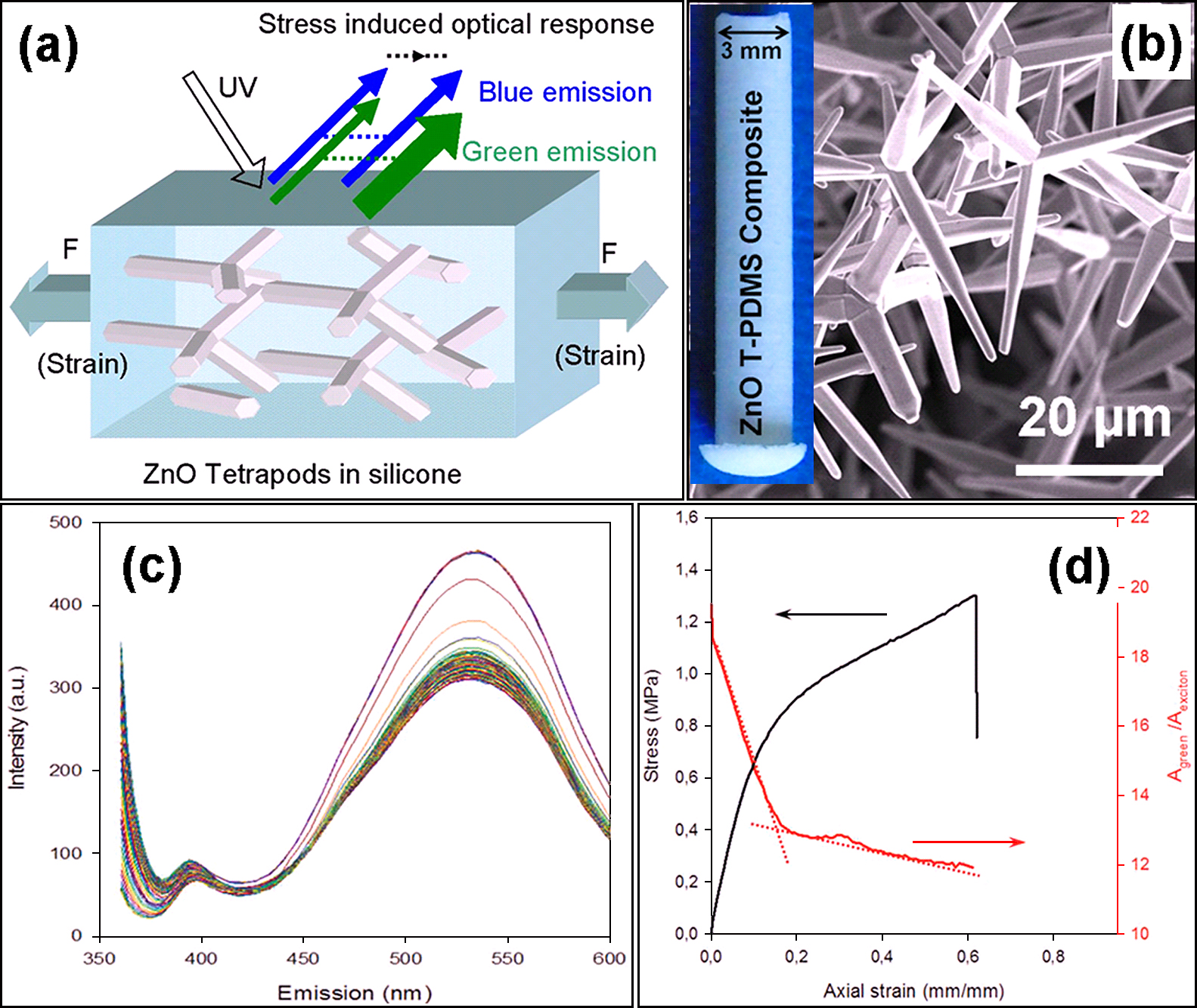
Use of luminescence features of ZnO tetrapods (T-ZnO) for designing self-reporting composites: (a) Schematic drawing showing the change in luminescent response from ZnO tetrapods under tensile stress. (b) SEM image of ZnO tetrapods. The inset in (b) shows a digital camera image of the fabricated T-ZnO:PDMS composite. (c) Photoluminescence spectra (raw data for plot d) from the T-ZnO: PDMS composite (50 weight % T-ZnO) under tensile stress (at every 2.5 s interval, 100 spectra were collected during tensile test and 85 spectra are plotted here in which a clear change of intensity of PL peak ∼ 523 nm is visible). (d) Stress-strain plot of the T-ZnO:PDMS (50 weight % T-ZnO) composite (left side) and variation of intensity ratio of green to blue PL emissions regions with respect to strain. [Reproduced with permission from65)].
A strong correlation between the tensile stress and green PL signal was observed as shown in Fig. 10d. Furthermore, the polymer was reinforced by the addition of tetrapodal microparticles, which was not the case in other stress-sensing mechanisms involving photoluminescent particles as fillers65). Detailed analysis revealed that the formation of network of tetrapodal micro-particles is important for such correlations and a detailed monitoring of intensity ratio one might be able to extract to damage informations inside the composites. Further details can be found in the article by Jin et al.65)
4.5 ZnO 3D networks as templates for growth of ultra light weight aerographite materialThe 3D interconnected metal oxide nano-microstructures networks are extremely important for practical applications. Next, we demonstrate an example of growing new carbon based Aerographite material (shown in Fig. 11), which is one of lightest solid materials so far67). The interconnected networks from ZnO tetrapods were used as templates inside CVD chamber for growth of aerographite material. It was observed that the material growth follows exactly the tetrapods morphology and the existing interconnections in the 3D ZnO network template. The hydrogen gas flowing in the CVD chamber plays main role in the transformation of ZnO into aerographite as it etches ZnO from the surface of ZnO tetrapods and simultaneously deposits carbon at this place67). It was reported that belt like early stage growth is most likely responsible for this type of transformation of ZnO tetrapods into aerographite material67). This is an ultralight weight material with lot of interesting physical and chemical properties suitable for different applications67). Just for demonstration, SEM images from the fabricated aerographite material and some of its properties are summarized in Fig. 11.
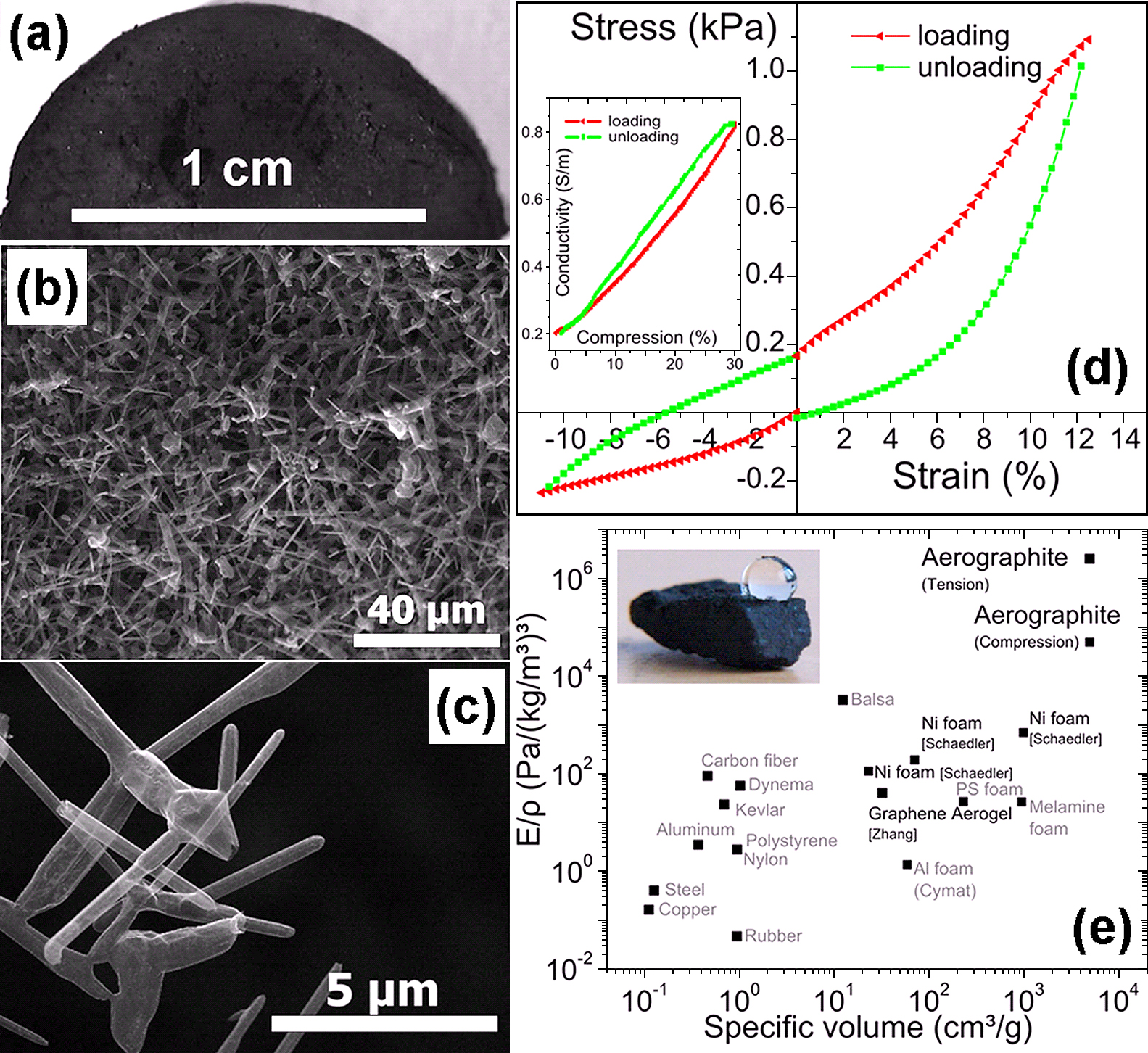
(a) Digital camera image of half-tablet of aerographite material. (b) & (c) SEM images of aerographite material at low (b) and high (c) magnifications. (d) Loading and unloading stress-strain response of aerographite demonstrates elastic behaviour under compressive and tensile stresses. During compression, a reversible increase of conductivity can be observed, see inset. (e) Specific modulus versus density map comparison of aerographite with respect to other light weight engineering materials. This materials selection map shows the theoretical superior properties of aerographite for the case of a minimized bending of a free standing bar with defined weight74). [Reproduced with permission from67)].
The 3D aerographite networks with desired sizes can be grown by proposed technique. For example digital camera image of a large cylindrical (1.4 cm diameter large with 1 cm height) aerographite network is shown in Fig. 11a and corresponding low and high magnification SEM images are shown Fig. 11b and Fig. 11c respectively. The high magnification SEM image (Fig. 11c) confirms the hierarchical hollow framework networks of microtubes inside aerographite network. Fig. 11e and d display mechanical data of aerographite material. In Fig. 11d, a mechanical load cycle containing compressive and tensile load is shown. The inset in Fig. 11d shows how the electrical conductivity increases during the mechanical compression in a similar experiment. Fig. 11e is an Ashby material selection map74) as it is a convenient guideline in modern lightweight engineering. It compares the specific modulus and density for a plenty of materials (including aerographite) because these two properties are very crucial to build stiff and light parts from an individual material. The here demonstrated Aerographite material exhibits interesting electromechanical properties (Fig. 11d) and material selection map (shown in Fig. 11e) suggests it to be a potential candidate for material engineering applications67). It is among one of the extremely porous materials (porosity > 99.9%) and still superhydrophobic in nature67). The aerographite material possesses a lot of other interesting features suitable for various advanced applications and more details can be found from the previous paper by Mecklenburg et al.67)
In conclusion, we point out that proposed flame transport synthesis FTS and its approaches offer really facile, cost-effective and scalable synthesis processes for versatile micro-nanostructures of various metal oxides. It also enables successful fabrication of different 3D interconnected networks with desired sizes, porosities, electrical conductivities etc. The tunable elastic modulus of these 3D networks in the range of rubber elastic region enables them as flexible ceramics for various technological applications. Three-dimensional interpenetrated micro-nanostructures can be used as appropriate networks for controlled growth different other structures for example and ultralight weight aerographite material. The nano-micro-scale ZnO tetrapods have already proved their promising applications in the direction of biomedical engineering, advance linking technologies and designing self-reporting composites etc. The controlled variant of FTS technology offers facile growth of quasi-1D nanostructures and their arrays. Preliminary experiments using ultralong single crystalline ZnO needles have shown promising features for applications in various fields. Most relevant applications of the metal oxide micro-nanostructures grown by FTS approach and its variants have been summarized in this paper and several others are under progress.
YKM sincerely thanks to Prof. Sotiris E. Pratsinis (Particle Technology Laboratory, ETH-Zurich) for kind invitation to write the article and to Ms. Natalie Winzer (HOSOKAWA ALPINE Aktiengesellschaft, Germany) for her enormous patience in terms of deadline extensions and reviewing. This project was funded by German Research Foundation (DFG) under the schemes SFB 855-A5 and partially by SFB 677-C10 and Ad/183/10-1. YKM and OL acknowledge the researcher fellowships from Alexander von Humboldt Foundation at the University of Kiel, Institute of Materials Science, Germany.
Yogendra Kumar Mishra
Yogendra Kumar Mishra is currently working as a senior research assistant and habilitant in the Functional Nanomaterials Group at the Institute for Materials Science in the University of Kiel, Germany. He received his Ph.D. in physics (plasmonics) from Jawaharlal Nehru University, New Delhi, in September 2008, and since October 2008 has been working in the Functional Nanomaterials Group. During February 2009 to February 2011, he worked as an Alexander von Humboldt fellow in the group. At Kiel, he introduced a new fabrication technique called ‘Flame Transport Synthesis’ which allows the versatile fabrication of different metal oxide nano-microstructures and their interconnected 3D networks.
Sören Kaps
Sören Kaps studied materials science at the University of Kiel and received his diploma in 2008. He joined the group of Prof. Rainer Adelung (Functional Nanomaterials) as a Ph.D. candidate in 2008. His research is focused on the application of ZnO in magneto-electric sensors as in the DFG project SFB 855 Biomagnetic Sensing.
Arnim Schuchardt
Arnim Schuchardt received his B.Sc. (materials science) in 2008 at the University of Kiel. In 2010 he received his M.Sc. (materials science) at the University of Kiel. Since 2010, he has been a Ph.D. student in the Functional Nanomaterials Group, Institute for Materials Science in the University of Kiel, Germany. He is mainly working on the fabrication and electromechanical characterization of highly porous carbon-based 3D networks (aerographite).
Ingo Paulowicz
Ingo Paulowicz is currently working as a Ph.D. candidate in the Functional Nanomaterials Group at the Institute of Materials Science, University of Kiel, Germany. He finished his M.Sc. in 2010 at the very same place on the synthesis and characterization of tin oxide nanostructures. He prepared his B.Sc. at Hochschule Bonn-Rhein-Sieg on the topic of laser-sintered titanium dioxide, which he finished in 2008. His present field of work is mainly the synthesis of zinc oxide, in particular via a modified burner approach synthesis (m-BAS).
Xin Jin
Xin Jin studied Physics at the East China Normal University in Shanghai. She obtained her Master’s degree (2010) in material science and engineering from the University of Kiel. Since 2010 she has been a Ph.D. candidate in the group of Prof. Dr. Rainer Adelung (Functional Nanomaterials), University of Kiel, Kiel, Germany. She is working mainly on the DFG project SFB677 which focuses on photoswitchable adhesives. She recently introduced the new concept of joining the un-joinable polymers employing ZnO tetrapods as linkers.
Dawit Gedamu
Dawit Gedamu is a Ph. D. candidate. in materials science at the Functional Nanomaterials Group, Institute for Materials Science, University of Kiel. His research interest mainly includes the synthesis of different metal and metal oxide nanowires and their potential for various nanoelectronic devices and sensors. He received an M.Sc. in material science and engineering in 2008 from the University of Kiel, Kiel, Germany.
Sebastian Wille
Sebastian Wille is currently working as a leader of the dental materials group in the Department of Prosthodontic, Propaedeutics and Dental Materials at the UKSH in Kiel on various projects about dental materials. A key topic is the aging of dental zirconia ceramics. He received a Ph.D. degree in materials science (Dr.-Ing.) from the University of Kiel in May 2009. He continued working in the group of Functional Nanomaterials in Kiel until December 2012 as a scientist, concentrating mainly on several projects about the application and synthesis of metal oxide micro- and nanostructures.
Oleg Lupan
Oleg Lupan is a research fellow supported by the Alexander von Humboldt Foundation at the University of Kiel, Institute of Materials Science, Functional Nanomaterials. He received his M.S. in microelectronics from the Technical University of Moldova (TUM) in 1993. He received his Ph.D. in solid-state electronics, microelectronics and nanoelectronics from the Institute of Applied Physics, Academy of Sciences of Moldova in 2005. His post-doctorate research activities were carried out at the CNRS, Paris, France, and the University of Central Florida, USA. He received his doctor habilitate degree in 2011. He is an associate professor at the TUM. His current research interests include nanosensors and optoelectronic devices.
Rainer Adelung
Rainer Adelung is a full professor and chairholder of the Functional Nanomaterials Group established in 2007 at the Institute for Materials Science, University of Kiel, Germany. He received his Ph.D. (rer. net) in physics in 2000 from the Institute of Experimental and Applied Physics, University of Kiel, and during 2001–2002, he was at Case Western Reserve University in Cleveland (USA) as a Feodor Lynen (Alexander von Humboldt) Research Fellow. In 2006 he finished habilitation at the Institute for Materials Science in Kiel and then continued as a Heisenberg Professor (DFG grant) with his own Functional Nanomaterials Group until 2012.