2018 Volume 35 Pages 49-65
2018 Volume 35 Pages 49-65
Nanoparticles have attracted much attention as a key material for new biomedical and pharmaceutical applications. For success in these applications, the nanoparticles are required to translocate across the cell membrane and to reach to inside of the cell. Among several translocation pathways of nanoparticles, the direct permeation pathway has a great advantage due to its high delivery efficacy. However, despite many research efforts, key properties and factors for driving the direct permeation of nanoparticle and its underlying mechanisms are far from being understood. In this article, experimental and computational studies regarding the direct permeation of nanoparticles across a cell membrane will be reviewed. Firstly, experimental studies on the nanoparticle-cell interactions, where spontaneous direct permeation of nanoparticles was observed, are reviewed. From the experimental studies, potential key physico-chemical properties of nanoparticles for their direct permeation are discussed. Secondly, physical methods such as electroporation and sonoporation for delivering nanoparticles into cells are reviewed. Current status of technologies for facilitating the direct permeation of nanoparticle is presented. Finally, we review molecular dynamics simulation studies and present the latest findings on the underlying molecular mechanisms of the direct permeation of nanoparticle.
With recent advances in nanoscience and nanotechnology, nanoparticles (NPs) have attracted much attention as a key material for the new biomedical and pharmaceutical applications. The NPs, defined as materials with at least one dimension in the size range smaller than 100 nm, exhibit significant unique properties that can be useful for the biomedical and pharmaceutical applications. The highly tunable size, structure, and surface properties of NPs at a nanometer scale can be exploited as carrier platforms for drug-, gene-, macromolecule-deliveries (Rana et al., 2012; Shao et al., 2015; Wilczewska et al., 2012). The fluorescence properties of semiconductor NPs can be used for bioimaging (Ekimov et al., 1985; Luo et al., 2012; Weller et al., 1986; Yuan et al., 2013). The unique plasmonic properties of metal NPs can be exploited for simultaneous imaging and photothermal therapies (Dreaden et al., 2011; Huang et al., 2006; Jaque et al., 2014; Kennedy et al., 2011; Périgo et al., 2015; Xiong et al., 2014). The magnetic properties of metal and metal-oxide NPs can be utilized for magnetic resonance imaging and magnetic fluid hyperthermia treatment for cancer therapy (Fortin et al., 2007; Veiseh et al., 2011). Meanwhile, many experimental investigations suggest that use of NPs can cause harmful effects: concerns about nanotoxicity have been elicited. It has been reported that NPs can cause cell membrane disruptions and significant increase in cell death at cellular level (Kahru and Ivask, 2013; Pietroiusti, 2012). NPs can also cause negative physiological effects such as inflammatory and immunological responses, resulting in adverse effects even at in vivo levels (Kim et al., 2013).
For success in the biomedical and pharmaceutical applications (i.e., for maximizing the NPs’ beneficial effects and minimizing their potential adverse effects), the NPs are required to be transported into the cell and localized at targeted cellular component without any damage to the cell. When the NPs are transported into the cell, the most critical barrier is the cell membrane. The cell membrane is a fundamental biological barrier and mainly composed of a lipid bilayer with membrane proteins. Transport of extracellular matters into a cell is selectively regulated by the cell membrane. Therefore, a key issue to realize the potential applications of NPs is development of a technology that can control the NP translocation across the cell membrane. To develop such a technology, understanding of mechanisms of the NP translocation across a cell membrane is necessary.
Through many research efforts up to now, it has been found that translocation of NPs across a cell membrane can be classified into two major pathways: endocytosis and direct permeation (Beddoes et al., 2015; Ding and Ma, 2015; Qu et al., 2013). Fig. 1 shows a schematic illustration of the NP translocation pathways. Endocytosis is an intrinsic cellular function to take up extracellular substances into the cell. In the endocytosis, a small portion of the cell membrane deforms and wraps extracellular NPs. Subsequently, the cell membrane pinches off and an endocytic vesicle which encloses the NPs is formed. The NPs enclosed by the endocytic vesicle are finally transported into the cell, leading to the NP translocation across the cell membrane. The direct permeation is defined as a non-endocytic translocation pathway. In this pathway, NPs permeates across the cell membrane without confinement of the NPs by the endocytic vesicles, leading to direct delivery of the NPs into the cell.

Translocation pathways of nanoparticles across cell membrane. Reprinted with permission from Ref. (Qu et al., 2013). Copyright: (2013) Future Science Group.
According to the reviews of experimental investigations (Beddoes et al., 2015; Qu et al., 2013; Zhu et al., 2013), it has been recognized that the endocytosis is a major translocation pathway when NPs interact with the cells, while the direct permeation is a minor one. However, the endocytosis possesses a significant drawback for the biomedical and pharmaceutical applications. The endocytosed NPs cannot often escape from the endocytic vesicles and cannot reach to the targeted cellular component even after translocating across the cell membrane (Ding and Ma, 2015). This leads to low delivery efficacy that is a critical issue for the biomedical and pharmaceutical applications. By contrast, in the direct permeation, NPs reach to inside of the cell without entrapment of NPs by vesicles, resulting in high delivery efficacy. This is a great advantage over the endocytosis, while the direct permeation is a rare event as compared to the endocytosis. To overcome this issue and facilitate the direct permeation, some physical methods, where external forces such as electric field or ultrasound are applied to the cell, are promising (Al-Dosari and Gao, 2009; Mehierhumbert and Guy, 2005). The physical methods were originally developed for gene transfer into cells. Recently, these are exploited for the direct delivery of NPs (Carrasco et al., 2016; Huang et al., 2014; Kawano et al., 2006; Kim et al., 2011; Lin et al., 2009; Wang et al., 2014; Yang et al., 2011; Zu et al., 2014).
Understanding of the mechanism of the direct permeation of NPs across the cell membrane with or without external forces can greatly contribute to realize an ideal NP delivery method with both high delivery efficacy and less damage to the cell. The direct permeation of NPs can be controlled by two potential key factors: physico-chemical properties of the NPs; nature and conditions of the external forces. However, despite many research efforts, it is still very challenging to control the direct permeation of NPs across a cell membrane. The key properties and factors for controlling the NP direct permeation and its underlying mechanisms are far from being understood. This lack of understanding hinders the development of new technology which can control the NP direct permeation, and also hinders the design of NPs with suitable properties for their applications.
In this article, the existing studies regarding the direct permeation of NPs across the cell membrane will be reviewed. Our aim is to present (i) potential key physico-chemical properties of NPs for their direct permeation; (ii) potential physical methods for facilitating the NP direct permeation; (iii) current understanding of the molecular mechanisms of the NP direct permeation. Firstly, experimental studies on the nanoparticle-cell interactions, where the direct permeation of NPs without applying external forces were reported, will be reviewed. From this review, we will extract insights into key physico-chemical properties of NPs for their direct permeation. Subsequently, potential physical methods such as electroporation and sonoporation for facilitating the NP direct permeation are reviewed. Finally, computational studies will also be reviewed, and the latest findings on the underlying molecular mechanisms of the NP direct permeation will be presented.
There are many factors that can affect the NP-cell membrane interactions. Among the potential key factors, physico-chemical properties of the NPs (e.g., size, shape, charge, hydrophobicity/hydrophilicity, surface chemistry, and others) can highly influence the NP-cell membrane interactions. Although there are some reviews on the key NP properties for the NP-cell membrane interactions including adhesion, endocytosis, and membrane disruption (Beddoes et al., 2015; Qu et al., 2013; Verma and Stellacci, 2010; Zhu et al., 2013), the potential key properties for the NP direct permeation have not been reviewed. Herein, we have surveyed numerous experimental studies and will discuss the potential key physico-chemical properties for their direct permeation across cell membranes. In this section, we will only focus on the NP direct permeation without applying external forces, i.e., passive permeation of NPs.
Table 1 shows a summary of experimental studies in which the direct permeation of NPs across a cell membrane was observed. First of all, size of the NPs can be an important factor. In general, for NPs in the size range from several tens to hundreds of nanometers, endocytosis is a major mode of NPs’ translocation across a cell membrane. However, for very small NPs, it can be considered that the endocytosis may be unfavorable. Yi et al. (2011) calculated an energy change resulting from the membrane wrapping of a single NP using a thermodynamic theory proposed by Helfrich (1973). Their results exhibited that a higher energy cost was required for the membrane wrapping of a smaller NP, while the membrane wrapping of a larger NP was energetically favorable. Thus, it is expected that for the smaller NPs the direct permeation can be favorable (Ding and Ma, 2015). In fact, as seen in Table 1, the size of NPs that exhibited the direct permeation is mostly less than 20 nm. Van Lehn et al. (2013) showed that even with very tiny decrease in the NP size from 5.8 nm to 2.4 nm, amount of NPs entering into cells by the direct permeation significantly increased. Jiang et al. (2015) also reported that the uptake amount of NPs through the direct permeation showed significant dependence on the NP size within a small range of 2 to 6 nm. Although there is no general consensus yet, the experimental results imply that for delivering NPs into cells by the direct permeation mode, size of the NPs seems to be desired to be smaller than 50 nm at least. NPs smaller than 10 nm are more preferred.
| Reference | Nanoparticle properties | Types of cell/ membranea | Culture/ dispersion mediumb and presence/absence of serumc | Comments | |||
|---|---|---|---|---|---|---|---|
| Material | Size (nm) | Surface modification | Surface charge/ potential | ||||
| Arvizo et al. (2010) | Gold | 10 | Ligand terminals with:
|
|
|
|
|
| Cho et al. (2009) | Gold | 18 |
|
|
SK-BR-3 |
|
|
| Geiser et al. (2005) | Gold | 25 | — | ― |
|
RPMI1640 with FBS | NP directly penetrated into both of cells |
| Goda et al. (2010) | Amphiphilic co-polymer | 10–12 | ― | 2 to 4 mV | HepG2 | DMEM with FBS | NP showed direct permeation |
| Hong et al. (2004, 2006) | Cationic dendrimers | 10–100 | Amine terminated surface | Positively charged |
|
|
NP formed holes on membranes, followed by direct permeation |
| Jewell et al. (2011) | Gold | 5 |
|
Negatively charged (both) | B16-F0 | Serum-free DMEM |
|
| Jiang et al. (2015) | Gold | 2–6 | Ligand terminals with:
|
|
Hela cell | Serum-free DMEM |
|
| Leroueil et al. (2008) | Gold | 5 | Alkylamine | Positively charged | Supported DMPC bilayer | NaCl aq. | NP formed holes on membrane, followed by direct permeation |
| Leroueil et al. (2008) | SiO2 | 50 | Amine terminated surface | Positively charged | Supported DMPC bilayer | NaCl aq. | NP formed holes on membrane, followed by direct permeation |
| Liu et al. (2013) | pNIPAM | 2700 | ― | ― |
|
|
Direct permeation was observed at an elevated temperature, inducing phase transition of NP from hydrophilic to hydrophobic |
| Lund et al. (2011) | Gold | 4 |
|
|
HCT-116, HT 29, LS154T, and SW640 | DMEM | NPs directly penetrated into cell |
| Mu et al. (2012) | SiO2 | 14 | ― | ― | A549 | DMEM with FBS | NP directly penetrated into cell |
| Nativo et al. (2008) | Gold | 16 | Coated with cell penetrating peptides | ― | Hela cell | DMEM with FBS | NP directly penetrated into cell |
| Rothen-Rutishauser et al. (2006) | Gold | 25 |
|
― | Human RBC | RPMI1640 with human serum | Both of NPs directly penetrated into cell |
| Rothen-Rutishauser et al. (2006) | TiO2 (anatase) | 20–30 | ― | ― | Human RBC | RPMI1640 with human serum | NP directly penetrated into cell |
| Van Lehn et al. (2013) | Gold | 2.4–5.8 | Anionic ligand | Negatively charged | Hela cell | ― |
|
| Van Lehn et al. (2013) | Gold | 2.2 | Anionic and neutral ligands | Negatively charged | DOPC vesicle | Sucrose aq. |
|
| Veiseh et al. (2011) | Iron oxide | 54 | Coated with PEG and poly-arginine | ― |
|
DMEM with FBS |
|
| Verma et al. (2008) | Gold | 5 |
|
|
DC2.4 | Serum-free RPMI640 |
|
| Wang et al. (2012) | CdSe/ZnS quantum dots | 8 | Zwitterionic ligand | ― | Human RBC | Serum-free PBS | NP directly penetrated into cell |
| Yu et al. (2007) | Silver | ― | Designed peptide | ― | NIH 3T3 | Serum-free DMEM | NP directly penetrated into cell |
The surface charge of NPs can also affect the NP-cell membrane interactions. Many studies regarding NP-cell interactions have pointed out that positively charged NPs are more likely to provide significant influence on the cell as compared to neutral or negatively charged NPs due to attractive electrostatic interactions with the negatively charged cell membranes (Beddoes et al., 2015; Cho et al., 2009; Verma and Stellacci, 2010). However, as shown in Table 1, the direct permeation of NPs has been observed regardless of the surface charge of the NPs: i.e., the NPs’ surface charge may not be determinant for the direct permeation. As seen in Table 1, a positively charged NP exhibited higher uptake through the direct penetration pathway than neutral and negatively charged NPs (Arvizo et al., 2010; Cho et al., 2009), while a negatively charged NP exhibited the direct permeation (Jewell et al., 2011; Lund et al., 2011; Van Lehn et al., 2013; Verma et al., 2008). Van Lehn et al. (2013) reported that the negatively charged gold NP directly penetrates into the synthetic vesicle without disruption of the vesicle membrane, while other studies reported that positively charged NPs can induce disruption of cell membranes, leading to cell death (Hong et al., 2004, 2006). Jiang et al. (2015) observed that the zwitterionic NP was primarily uptaken by the direct permeation, while the positively and negatively charged NPs were primarily uptaken by endocytic pathways. In summary, from the current experimental findings it can be considered that the NPs’ surface charge is not likely to be a determinant of the NP direct permeation.
Hydrophobicity/hydrophilicity of the NPs can be an important property, because the cell membranes are composed of amphiphilic phospholipid molecules. Goda et al. (2010) found that their synthesized NP composed of amphiphilic random copolymer exhibited the direct permeation, while another NP composed of solely hydrophilic polymer failed to penetrate into the cell. Liu et al. (2013) reported that temperature sensitive pNIPAM particles with a size of 3 μm, which is much larger size, can directly penetrate into phospholipid vesicles and Hela cells. They considered that this is due to phase transition of the pNIPAM from hydrophilic to hydrophobic at an elevated temperature above its phase transition criterion. From these studies, amphiphilic nature seems to be favorable for the direct permeation, although further investigations are necessary.
Surface modification of NPs is effective to control the NP direct permeation. As seen in Table 1, variety of functional groups, ligands, biomolecules, polymers have been investigated as surface modifiers to facilitate the NP direct penetration. It has also been found that not only types of modifiers but also their structural arrangement can be a key factor for the NP direct permeation. Verma et al. (2008) synthesized two kinds of gold nanoparticles coated by anionic and neutral ligands with same size, same surface charge, and same ligand composition. One particle was coated in an ordered striped arrangement of anionic and neutral ligands, while the another particle was coated with the same two ligands in a random arrangement. They found that the former particle with the ordered arrangement can directly permeate across the cell membrane without the membrane disruption, whereas the latter particle with the random arrangement was mostly trapped in endosomes by the endocytosis. A similar result has been observed in Jewell et al. (2011). A possible explanation on this effect is that the ligand pattern in the ordered arrangement may be assumed to be more rigid, resulting in the direct permeation across the cell membrane (a more fluid layer) without the membrane disruption, which is similar mechanism for cell penetrating peptides (Verma et al., 2008).
With regard to material of the NPs, it seems that gold is likely to exhibit the direct permeation across cell membranes as compared to other materials (Table 1). However, we are speculating that this is not attributed to the material but to the size requirement for the direct permeation. Size of the gold NPs is generally highly tunable. Synthesis of gold NPs smaller than 10 nm is facile as compared to other materials. This may be a possible reason behind the specificity of gold shown in Table 1.
The membrane properties can be important factors for the NP direct permeation. Moreover, external environmental conditions such as pH, osmotic pressure, ionic strength, and presence of serum proteins can also be important factors. However, influences of these factors on the NP direct permeation have not been investigated.
For direct delivery of extracellular objects into the cell, some physical methods have been developed. The physical methods here refer to physical manipulations to improve the efficacy (rate and extent) of the delivery of extracellular objects. Over the past two decades, the following physical methods have been developed for the gene delivery into cells: electroporation; sonoporation; laser irradiation; magnetofection; etc. (Al-Dosari and Gao, 2009; Mehierhumbert and Guy, 2005). In these methods, the cells are exposed to each type of external force fields, forming transient defects and pores in the cell membrane. This results in temporal enhancement of permeability of the cell membrane, leading to direct permeation of extracellular objects in the surrounding medium across the cell membrane. Meanwhile, an increase in cellular mortality can often be a great concern in these physical methods, if cells are subjected to the excess stress derived from the applied external forces. With regard to the direct delivery of NPs, the electroporation (Huang et al., 2014; Kawano et al., 2006; Kim et al., 2011; Lin et al., 2009; Zu et al., 2014) and sonoporation (Carrasco et al., 2016; Wang et al., 2014; Yang et al., 2011) have been utilized in recent. In the following, these studies will be reviewed.
Electroporation is a technique where an electric field (a high intensity electric pulse) is applied to cells. The electric pulse can cause local destabilization of the cell membranes followed by electric field-induced transient pores, resulting in the direct delivery of extracellular objects into the cell. More details of fundamental physics of the electroporation can be found in many literatures (e.g., Al-Dosari and Gao, 2009; Gehl, 2003; Kotnik et al., 2015; Mehierhumbert and Guy, 2005). The electroporation treatment is able to be applied to cells adhered on a culture dish as well as cells suspended in a culture media. Several types of electrodes for both types of cells have been developed and commercially available.
Recently, the electroporation has been utilized for delivery of NPs into cells. These NPs include DNA-conjugated gold NPs with 100 to 200 nm diameter (Huang et al., 2014; Kawano et al., 2006; Zu et al., 2014), silver NPs with 80 nm diameter (Lin et al., 2009), and hollow metal NPs coated with mesoporous silica layer with 65 nm diameter (Kim et al., 2011). These experimental studies demonstrated significant enhancement of delivery efficacy of NPs with the electroporation treatment. Huang et al. (2014) and Zu et al. (2014) showed that with the electroporation treatment the dominant NP delivery pathway into cells shifted from the endocytosis to the direct permeation. It should be noted that even NPs with relatively large particle size (ca. 100 nm) can be directly delivered into cells through the direct permeation pathway using the electroporation. However, negative impacts on cellular functions such as viability and cell differentiation were somewhat found after the electroporation (Huang et al., 2014; Kim et al., 2011). To overcome this disadvantage, some efforts have been reported. Zu et al. (2014) found that both of high delivery efficacy of DNA-conjugated NPs and low cell mortality can be achieved by adding pristine gold NPs as an additive. They considered that this is due to decreasing the electric resistance of the medium with highly conducting gold NPs, resulting in much lower applied intensity required for the delivery. A new electroporation system integrated with microfluidic devices has also been proposed (Geng and Lu, 2013; Wang et al., 2010). In the microfluidic electroporation system, a single cell can be manipulated and be exposed to uniform electric field, as compared to conventional batch electroporation where electric pulse is applied to a bulk cell suspension. This can provide a precise control of electric field per single cell, leading to improvement on the delivery efficacy and less cellular damage.
In order to realize an ideal electroporation-assisted NP delivery with high delivery efficacy and less cellular damage, it is necessary to understand the mechanism underlying the NP permeation in an electric field. In particular, role of NPs’ physico-chemical properties on the mechanism of the NP permeation remains subject of investigation. Elucidation of this mechanism can greatly contribute to optimize design of the NPs and operating conditions of the electroporation (e.g., strength and frequency of the electric current, number of electric pulses, and so on).
Sonoporation is a technique where an ultrasound is applied to a suspension of cells and microbubbles. It has been considered that an acoustic cavitation is a central cause of enhancement of the permeability of the cell membranes. Acoustic cavitation generated by the ultrasound irradiation can cause generation, oscillation, and collapse of active bubbles. This results in a release of associated mechanical stresses such as radiation force, micro-streaming, micro-jets, or shock waves, leading to the transient pore formation across adjacent cell membranes. According to this mechanism, the ultrasound-mediated bubbles are necessary for the sonoporation. In clinical applications, ultrasound contrast agents consisting of microbubbles stabilized by surface active molecules such as proteins, polymers, and lipids have often been used for the sonoporation-assisting agents. More details of fundamentals and historical development of the sonoporation can be found in many literatures (e.g., Al-Dosari and Gao, 2009; Kodama et al., 2006; Mehierhumbert and Guy, 2005; Zhou et al., 2014).
Recently, sonoporation-assisted NP deliveries have been investigated. Carrasco et al. (2016) employed a shock wave irradiation for delivery of mesoporous silica NPs into human embryo kidney cells. Although no microbubble additives were used, they verified that the shock wave irradiation significantly enhanced the delivery of the mesoporous silica NPs with 200 nm diameter. Another approach in which NPs/microbubble composites are synthesized and utilized for better delivery of NPs has also been investigated. Wang et al. (2014) synthesized a gold nanorod (AuNR)-microbubble composite, in which AuNRs were encapsulated in perfluorocarbon gas filled microbubbles (3 to 5 μm diameter) covered with a protein/antibody shell. Amount of the AuNRs delivered into tumor cells showed significant increase when the AuNR/microbubble composite was used with a sonoporation treatment as compared to the control condition (without encapsulation of AuNRs in microbubbles). This study suggests that encapsulation of NPs in microbubbles can enhance the delivery performance of NPs in the sonoporation treatment. Yang et al. (2011) synthesized NP-embedded microbubbles composite in which Fe3O4 nanoparticles (12 nm diameter) were embedded in a polymer layer covering microbubble (3 to 5 μm diameter). They showed that amount of Fe3O4 nanoparticles delivered into tumor cells significantly increased by the ultrasound irradiation. They also found that the Fe3O4-embedded microbubbles exhibited higher cell viability as compared to a control condition under the same ultra sound intensity. This suggests that NPs/microbubble composites can reduce damage to the cells associated with the sonoporation.
Many questions and issues still remain unsolved in the sonoporation-assisted NP permeation. Operating conditions of the sonoporation treatment (e.g., intensity, frequency, duration of the ultrasound irradiation) can be key factors. Wang et al. (2014) and Yang et al. (2011) suggested that NP delivery rate can be controlled by the intensity and number of the ultrasound irradiation. Physico-chemical properties of NPs can also be important factors. Carrasco et al. (2016) found that a surface coating of NP with cationic lipid molecules attenuate the decrease in the cell viability after the sonoporation treatment. Interaction between NPs and microbubble is also important subject of investigation, when NPs/microbubble composites will be employed. Role of these potential key factors should be investigated to optimize the sonoporation-assisted NP permeation.
Understanding of the mechanism of the direct permeation of NPs across a cell membrane can greatly contribute to synthesizing new NPs and to developing a new NP delivery technique with high delivery efficacy and less cellular damage. However, the molecular mechanism underlying the NP direct permeation is poorly understood. Although a lot of experimental studies on the NP–cell membrane interactions have been conducted, it is still difficult to investigate the molecular mechanism using current experimental techniques. One of the potential ways to investigate the NP–cell membrane interactions is a computational modeling. In particular, molecular dynamics (MD) simulations can be a powerful approach. In the MD simulations, trajectories of individual atom can be calculated so that dynamic evolution of the system consisting of the NP, lipid molecules, and solvents can be simulated at the molecular scale. This can provide deep insights into the understanding of the NP-cell membrane interactions. Coarse-grained MD methods have often been adapted for the NP-cell membrane interactions. In the coarse-grained MD methods, small groups of atoms are represented by a single interaction sites as coarse-grained sites. This can greatly reduce the computational load and enables to simulate the NP-cell membrane interactions with appropriate system size (larger than ten nanometers) and length of time (longer than a few hundred nanoseconds). More details of the MD simulation methods for the NP-cell membrane interactions can be found in the literatures (Ding and Ma, 2015; Qu et al., 2013; Rossi and Monticelli, 2016).
We surveyed MD simulation studies reported so far, and simulated final fates of NPs after interacting with a lipid bilayer (model cell membrane) will be reviewed. Fig. 2 shows summary of the final fates of NPs reported in previous studies. The MD simulation studies suggest that the final fates of the NPs will become either adhesion, penetration, or wrapping (Fig. 2(a)). Here, it should be noted that the further permeation of the NPs across the membrane cannot be observed unless applying external force on the NP (Fig. 2(b)) or adopting some additional settings (Fig. 2(c)). This review is focusing on the direct permeation, thus, we will review the MD simulation studies which can be classified into groups of Fig. 2(b) and (c). We here define the terms to distinguish between these two groups: “biased” and “unbiased” MD simulations. The biased MD simulations correspond to Fig. 2(b), where a NP was artificially forced to permeate across the membrane. Although this is far from a reality, the biased MD simulations can provide insights into that how the NPs can be designed to achieve high permeability and low cellular damage. On the other hand, the unbiased MD simulations correspond to Fig. 2(c), where any external forces are not exerted on the NP, but other additional settings are adopted. The unbiased MD simulations can provide insight into physical requirements for the direct permeation of NPs across a lipid bilayer. In the following sections, the biased and unbiased MD simulation studies will be reviewed, respectively.

Summary of final fates of nanoparticles after interacting with lipid bilayer reported in MD simulation studies.
As mentioned above, the biased MD simulations, where the NP is forced to permeate across the membrane by applying an external force to the NP, have been conducted for investigating potential key NP properties for the NP direct permeation across a cell membrane. The permeation process has been characterized by physical and thermodynamic properties such as the minimal driving force required for the NPs to permeate lipid bilayers and the potential of mean force (i.e., the free energy change) along the NP permeation across the cell membrane.
Song et al. (2011) investigated influence of NP size on the direct permeation across a phospholipid bilayer by means of the biased MD simulation. They used gold NPs in the range of 0.8 to 2.5 nm diameter and with nearly spherical shape. They calculated the minimum driving force required to permeate a DPPC bilayer at various NP sizes. With a decrease in the size of gold NP, the driving force was smaller. This result is similar with an experimental observation presented in Section 2: the smaller the nanoparticle, the easier it is for the direct permeation. The minimum force for permeating whole bilayer (both first and second monolayers) was ca. 100 to 500 pN in the range of the size of 0.8 to 2.5 nm. Song et al. (2011) also reported that the minimum force calculated from their unbiased MD simulations showed an agreement with an experimental result obtained from an atomic force microscopy (Vakarelski et al., 2007), although further investigations seem to be needed. They also calculated the minimum driving pressure, which was defined by the minimum force per cross-sectional area of the NP. They found that the pressure required to permeate whole bilayer deceased with an increase in the nanoparticle size.
Shape of the NP is another key property. Some computational modeling studies on the role of particle shape in the NP permeation have been performed. From the previous studies, a consensus has been addressed: the sharp shape can be advantageous for the NP direct permeation. Yang and Ma (2010) performed a MD simulation of the NP permeation across a lipid bilayer (Fig. 3(a)). They calculated the minimum driving force of NPs with various shapes (Fig. 3(b)) by means of the biased MD simulation. Their result (Fig. 3(c)) revealed that the higher minimum driving force was required at larger radius r (i.e., larger contact area between particle and bilayer), while the minimum driving force was less sensitive to the particle height h. Interestingly, the minimum driving force significantly decreased even at higher particle height in the case of the V-shaped particle (the green circle plot in Fig. 3(c)), where the local curvature at the contact surface between the NP and bilayer was much higher and the disruption area due to the particle permeation gradually increased as the NP permeated into the membrane. The authors concluded that the permeability of nanoparticles with anisotropic shapes can be determined by the contact area and the local curvature: i.e., the direct permeation can be more likely to occur at the smaller contact area and the higher local curvature. They also demonstrated that the initial orientation of the NP toward the lipid bilayer strongly relates to the capability of the NP direct permeation across the lipid bilayer. Shi et al. (2008) investigated permeation behavior of carbon nanotubes (CNTs) with different tube diameters. Their simulation result showed the tube diameter can be a determinant of the permeation mode of the CNTs: a CNT with smaller tube diameter (single-walled CNT) can directly permeate the bilayer via a piercing mode, while a CNT with larger tube diameter (multi-walled CNT) is likely to translocate the lipid bilayer via a wrapping mode. Moreover, they proposed theoretical models for the piercing and wrapping modes based on a vacancy diffusion theory and a fluid mechanics theory for an imcompressible viscous fluid. As a result, they successfully explained that the dominant permeation mode can switch from the piercing to the wrapping at a critical radius as the tube radius increases.

(a) Permeation behavior of elongated nanoparticle in biased MD simulation. (b) Various particle shapes and (c) minimum driving force for their direct permeation across lipid bilayer. Reprinted with permission from Ref. (Yang and Ma, 2010). Copyright: (2010) Nature Publishing Group.
In most of the biomedical and pharmaceutical applications utilizing NPs, surface modifications of the NPs have been frequently conducted to modify surface properties such as surface charge, hydrophilicity/ hydrophobicity, and specific interactions with cells. This can control the NP translocation across the cell membrane. Some biased MD simulation studies have investigated that how surface modifications of NP affect its permeation across a lipid bilayer. Song et al. (2012a) performed a MD simulation of permeation behavior of ligand-coated nanoparticles across a DPPC bilayer. They used alkanethiol-coated gold nanoparticles with different alkyl chain lengths. By means of a biased MD simulation, influence of the ligand length on the minimum driving force to permeate the bilayer was investigated. With an increase in the ligand length, the minimum driving force for permeating the first layer of the lipid bilayer was reduced, while the higher driving force was required to permeate the second layer and whole lipid bilayer. This is due to the favorable interactions between the hydrophobic lipid tails and hydrophobic ligands on the NP. This hydrophobic interaction becomes higher at longer ligand length, resulting in reducing the minimum force for permeating the first layer (from hydrophilic to hydrophobic) and increasing the minimum force for permeating the second layer (from hydrophobic to hydrophilic). As presented in Section 2, some experimental studies exhibited that the NP direct permeation can be enhanced by surface modifications with orderly arranged ligand structural pattern (Jewell et al., 2011; Verma et al., 2008), while its mechanism is still unknown. This issue has been investigated by MD simulation studies. Li et al. (2012) investigated the direct permeation of ligand-coated NPs with two different types of ligand structural patterns: striated pattern with orderly arranged hydrophilic and hydrophobic domains (“St” in Fig. 4(a)), and randomly arranged pattern at the same ratio of the hydrophilic and hydrophobic ligands (“Rand” in Fig. 4(a)). The simulation results demonstrated that the ligand structural pattern significantly affects the critical force for permeation of NPs across the lipid bilayer, and the striated ligand pattern results in the lowest critical force (Fig. 4(b)). They also investigated the free energy change associated with the permeation of NPs across the lipid bilayer, (Fig. 4(c)). The NP with random ligand pattern encountered substantial energy minimum at the center of bilayer, while the NP with striated ligand pattern flattened the energy minimum. This means that NP with striated ligand pattern is likely to permeate the lipid bilayer as compared to the NP with random ligand pattern, which can be trapped in the hydrophobic region of the bilayer. The similar free energy change induced by switching from random ligand structure to ordered ligand structure has been also reported by Gkeka et al. (2013). Li et al. (2012) conducted further analysis for understanding why the NP with striated ligand pattern exhibited the higher permeability. They indicated that the shallow energy minimum of the striated NP is attributed to lower degree of freedom for rotation of the striated NP in the hydrophobic region of the bilayer. The rotation of the striated NP during permeation across the bilayer was found to be constrained as compared to the NP with random ligand pattern due to the anisotropic ligand pattern. This prevented the NP from further fitting with the hydrophobic tails of lipid molecules, avoiding to be trapped into a deeper energy minimum when the NP passes across the hydrophobic region of the lipid bilayer.

(a) Ligand-coated nanoparticles with different surface structural patterns. (b) Critical force for permeating lipid bilayer at each nanoparticle with different surface ligand patterns. (c) Free energy change associated with permeation of various nanoparticles with different surface ligand patterns. Reprinted with permission from Ref. (Li et al., 2012). Copyright: (2012) The Royal Society of Chemistry.
Although biased MD simulations reported so far have provided deep insights into the molecular mechanism of the NP direct permeation across cell membranes, many aspects of the mechanism are still unknown. For example, role of physicochemical properties of the NPs such as surface charge and mechanical properties (soft/rigid) on the direct permeation across a cell membrane should be further investigated. Although many MD simulation studies regarding these properties have been reported, most of the previous studies have not focused on the permeation, while they have focused on adhesion or penetration into a lipid bilayer. Impact of the membrane properties such as composition of lipid molecules, bilayer phase, charge property, membrane surface tension should also be analyzed using a biased MD simulation. Moreover, not only the permeability of NPs but also changes of the lipid bilayer’s properties are important subjects to be investigated. From biased MD simulations, it has been observed that significant structural changes of the lipid bilayer such as pore formation and lipid flip-flop were induced by the NP permeation, followed by excessive leakage of waters and ions across the lipid bilayer (Oroskar et al., 2015; Song et al., 2012b). Such membrane disruption induced by the NP permeation should be analyzed further, providing important insights into the cellular damage and toxicity caused by the NP permeation.
4.2 Unbiased MD simulation studiesAs mentioned before, the MD simulation studies suggest that NPs cannot permeate across a lipid bilayer unless exerting an external force to the NP. However, experimental studies demonstrated that NPs can directly permeate across a cell membrane without exerting external force on the NP. Thus, a fundamental question remains unsolved: what are the physico-chemical requirements leading to the NP direct permeation? A few unbiased MD simulation studies have investigated to solve this issue. These unbiased MD simulations demonstrated that when some additional settings are adopted, an NP can directly permeate across a lipid bilayer. So far, the following special settings have been found to induce the NP direct permeation: the reversible reaction between NP and ligands (Ding et al., 2012), multiple NPs (Li et al., 2013), application of an external electric field (Shimizu et al., 2016).
Ding et al. (2012) proposed a new type of ligand-coated NP and demonstrated that their NP can directly permeate across a lipid bilayer (Fig. 5). Their ligand-coated NP consists of hydrophilic rigid core and amphiphilic ligands. As their uniqueness, they adopted a reversible reaction between the core particle and the ligands. In an aqueous environment, the hydrophilic head of the ligand binds onto the surface of the core particle. This results in the hydrophobic surface of the ligand-coated NP before interacting with a lipid bilayer. Due to this hydrophobic nature, the NP can spontaneously penetrate into the hydrophobic region of the membrane at first. Meanwhile, the ligand starts to break off in the hydrophobic region of the membrane due to reversible reaction responding the hydrophobic environment surrounding NP. Once the ligands leave from surface of the core particle, the hydrophilic surface of the core particle is exposed. Since the hydrophilic nature is unfavorable to stay in the hydrophobic region of the membrane, the NP is spontaneously pushed away and finally permeates across whole lipid bilayer. Although it is actually hard to synthesize such type of ligand-coated NPs, this study provides deep insight into a strategy of the particle design for the NP direct permeation pathway.

Direct permeation behavior of ligand-coated nanoparticle by adopting a reversible reaction between nanoparticle and ligands. Reprinted with permission from Ref. (Ding et al., 2012). Copyright: (2012) American Chemical Society.
In reality, number of NPs interacting with a cell membrane is huge. Moreover, the NPs may form an aggregation, and this aggregation may interact with a cell. This situation can be very different from a situation when the single primary NP interacts with a cell membrane. Thus, understanding of the NP-cell membrane interactions when there are multiple NPs is an important issue. Li et al., (2013) performed an unbiased MD simulation study on the interactions of peptide-conjugated NPs with a lipid bilayer (Fig. 6). The simulation result showed that when the NP interacted with a bilayer as a single particle any permeation of the NP was not observed. However, they found that when multiple NPs interact with a bilayer some NPs can permeate across the bilayer (Fig. 6). This NP permeation was induced by a pore-mediated process: i.e., multiple NPs cooperatively interact with a membrane and form a transmembrane pore. This is the similar mechanism of the transmembrane pore formation induced by multiple antimicrobial peptides (Leontiadou et al., 2006). Such cooperative interaction can be one of the potential mechanisms of the NP direct permeation.

Interaction of multiple peptide-conjugated nanoparticles with a lipid bilayer. Core of the nanoparticle is in red. The peptides are in yellow and pink. For the clarity, the peptides are not displayed at 130 ns, 1500 ns, and 2400 ns. Reprinted with permission from Ref. (Li et al., 2013). Copyright: (2013) The Royal Society of Chemistry.
As reviewed in Section 3, application of external force fields can be an effective way to directly deliver NPs into a cell, although its mechanism is largely unknown. We focused on the electroporation-assisted NP delivery and investigated for the first time the NP permeation across a lipid bilayer under an external electric field by means of an unbiased MD simulation (Shimizu et al., 2016). We used an alkanethiol-coated cationic gold nanoparticle with 4 nm diameter for the simulation. This is a typical NP for the biomedical and the pharmaceutical applications, and the direct permeation of this type of NP across a cell membrane have been observed (Table 1). Our study will be introduced in the following.
First of all, we investigated the NP behavior without the external electric field. The final fate of the NP was adhesion on the hydrophilic surface of the lipid bilayer, and the NP permeation across the bilayer was not observed (Fig. 7(a)). We also performed a biased MD simulation, i.e., the NP was forced to move downward across the bilayer by applying external force, while the electric field was not applied. Even in this case, the NP did not permeate across the bilayer, and partial wrapping of the NP by the bilayer was observed (Fig. 7(b)). Consequently, it was confirmed that the NP cannot permeate across a lipid bilayer without an external electric field. However, a drastic change of the NP behavior was observed when an external electric field was applied: the NP directly permeated across the lipid bilayer (Fig. 7(c) and (d)). Detail of this permeation process was as follows: (i) the NP adhered onto the hydrophilic surface of the bilayer; (ii) the NP penetrated into the bilayer while forming a hydrophilic pore in the membrane around the periphery of the NP; (iii) the NP finally permeated across the whole bilayer through the hydrophilic pore without membrane wrapping and pulling out of lipid molecules. Interestingly, as shown in Fig. 7(c), this NP direct permeation can be induced even under a lower applied membrane potential than the membrane breakdown potential. This implies that prior application of excessive electric field to form transmembrane pores before delivering NPs may not be necessary. Moreover, under a lower membrane potential than the membrane breakdown intensity (Fig. 7(c)) the transmembrane pore generated by the NP permeation immediately resealed after the NP permeation, while the persistent transmembrane pore was formed under an excessive membrane potential that is equal to the membrane breakdown potential (Fig. 7(d)). We also evaluated degree of the membrane disruption caused by the NP permeation by analyzing the lipid flip-flop and permeation amount of waters and ions across the membrane. The degree of the membrane disruption in the direct permeation pathway with self-resealing of the membrane (Fig. 7(c)) was significantly less and negligible as compare to the direct permeation with persistent transmembrane poration (Fig. 7(d)).
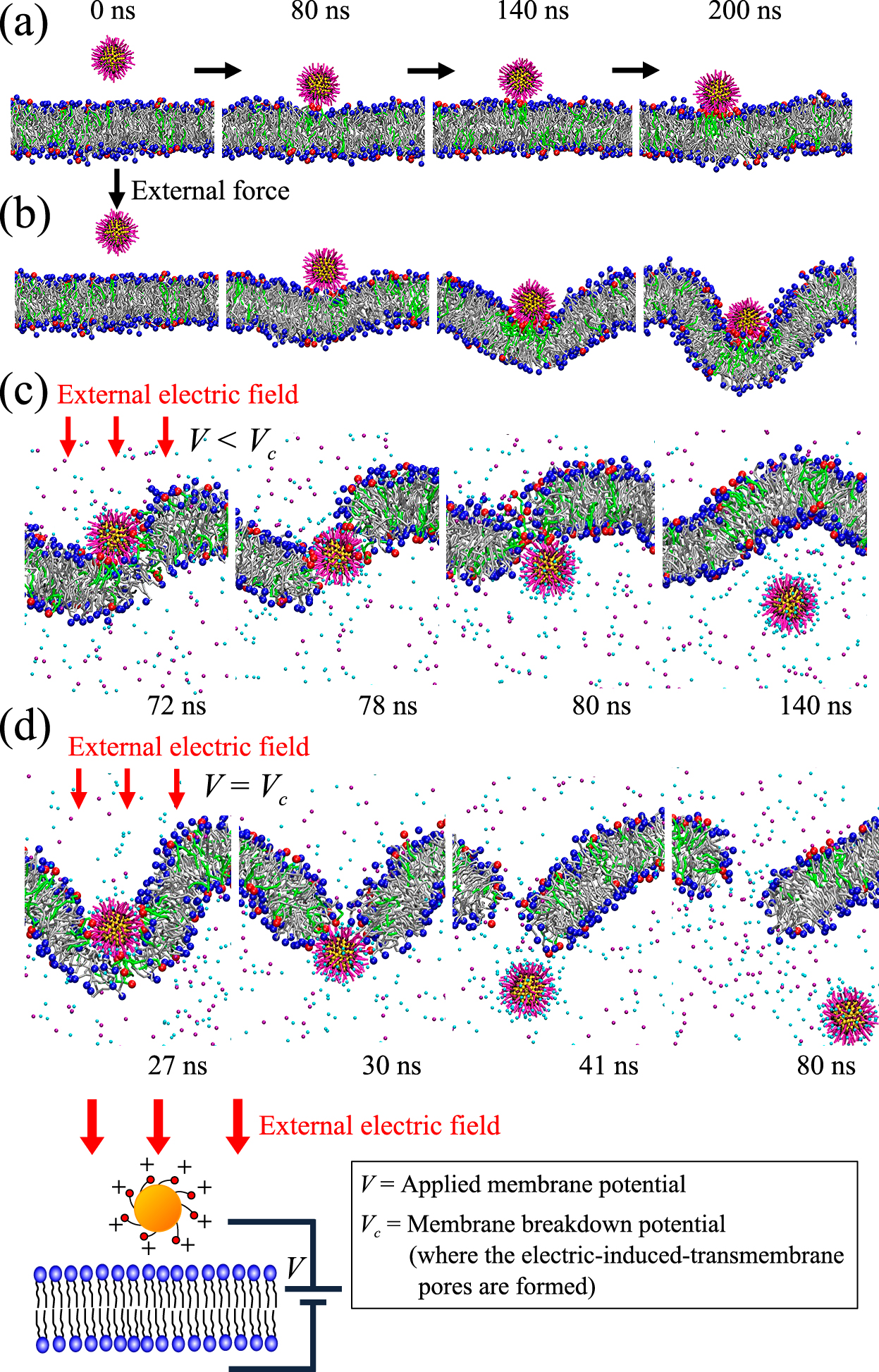
(a) Nanoparticle behavior without both external force on particle and electric field. (b) Nanoparticle behavior with external force on particle but without external electric field. (c) Nanoparticle behavior with external electric field but without external force on particle. Membrane potential that lower than the membrane breakdown intensity was applied (V = 0.8Vc). (d) Nanoparticle behavior under excessive applied membrane potential that is equal to the membrane breakdown intensity (V = Vc). Waters were not shown for clarity in (a) to (d). Ions were not shown in (a) and (b). Reprinted with permission from Ref. (Shimizu et al., 2016). Copyright: (2016) The Royal Society of Chemistry.
To investigate a key factor in the direct permeation of the NP, combined effects of the intensity of applied membrane potential and affinity of the NP for the membrane surface were investigated. Unbiased MD simulations using three different lipid bilayers (LB-1, LB-2, and LB-3 in Fig. 8) were conducted. Through the potential-of-mean-force analysis, we confirmed that the NP used in our study has a strong affinity with surface of the LB-3 (DPPC/DPPG leaflet), while less affinity with surfaces of the LB-1 and LB-2 (DPPC leaflet). We then conducted systematic unbiased MD simulations in each lipid bilayer under various applied membrane potentials. Fig. 8 shows a summary of the results. Following four modes of the NP behaviors were observed: (a) no adhesion; (b) adhesion on the membrane surface; (c) direct permeation with self-resealing of membrane; (d) direct permeation with persistent transmembrane poration. As shown in the result, occurrence probability of these four modes can strongly depend on affinity of the NP with membrane as well as applied membrane potential intensity. In particular, when we focused on the direct permeation pathway with self-resealing of membrane (the blue bar in Fig. 8), this ideal pathway is likely to occur in the LB-3: i.e., the case in which there is a strong affinity between the NP and surface of the membrane. The strong affinity can result in exclusion of the water and ions at the interface between the NP and the hydrophilic head group of the membrane. This leads to less remaining water and ions in the transmembrane pore during the NP permeation, preventing nucleation of the persistent hydrophobic pore and enhancing the self-resealing of the membrane after the NP permeation. In summary, our finding from the unbiased MD simulation with external electric field suggests that by controlling the external electric field as well as the NP surface properties in a suitable range, an ideal NP delivery pathway, where the NP can be directly delivered into the cell with high delivery efficacy and less cellular damage, can be achieved.

Occurrence probabilities of four modes of NP behaviors as a function of dimensionless applied membrane potential at different affinities between NP and membrane surface. V is applied membrane potential. Vc is the membrane breakdown potential. Results were within five independent simulation runs. Reprinted with permission from Ref. (Shimizu et al., 2016). Copyright: (2016) The Royal Society of Chemistry.
Our study can also provide insights into the issue of what the physico-chemical requirements can lead to the NP direct permeation. One of the key requirements may be “gradient” across the cell membrane. In actual NP-cell system, many intrinsic gradients such as pH difference, osmotic pressure, ionic concentration, resting transmembrane potential can be observed between outside and inside of the cell. These gradients can be a key for the NP direct permeation. Lin and Alexander-Katz (2013) demonstrated that a NP can directly permeate across a lipid bilayer under a transmembrane potential induced by the ionic imbalance across the bilayer. It is also well known that lipid composition of the two leaflets of the biological cell membrane is highly asymmetric. This asymmetric lipid composition can be a potential key property for the NP direct permeation. Role of these gradients and its combined effects with the NP physico-chemical properties in the NP direct permeation across a cell membrane should be investigated in further studies.
In this article, we focused on the direct permeation of nanoparticles (NPs) across a cell membrane and reviewed the related literatures on both experimental and modeling studies. First of all, we surveyed experimental studies where a spontaneous direct permeation of NPs across a cell membrane was observed, and reviewed the potential key physico-chemical properties of NPs for the direct permeation (Section 2). The experimental studies suggest that size, hydrophobicity/hydrophilicity, and structural arrangement of surface ligands can be critical NP properties for the direct permeation: much smaller size (smaller than 10 nm), amphiphilic nature, and ordered ligand pattern can be favorable for the NP direct permeation. However, a general consensus of the NP properties is not yet obtained and further investigations are necessary. Subsequently, potential physical methods for facilitating the NP direct permeation were surveyed (Section 3). Among them, electroporation- and sonoporation-assisted NP delivery were reviewed. Many studies demonstrated that by utilizing these physical methods a variety of NPs can be directly delivered into cells and the delivery efficacy of NPs can be significantly enhanced, while cellular damages are always concerned. To overcome this negative impact, it is necessary to investigate the mechanism underlying the NP permeation under external force fields. In particular, role of NPs’ physico-chemical properties on the mechanism of the NP direct delivery by the physical methods is an important issue to be solved. Finally, MD simulation studies on the NP permeation across a lipid bilayer were reviewed (Section 4). From review of the biased MD simulations, potential key NP properties for the direct permeation were discussed. The size, shape, and structural arrangement of surface ligands can be key properties for the direct permeation, although many aspects of the mechanism are still unknown. From the unbiased MD simulations, potential physico-chemical requirements leading to the NP direct permeation were reviewed. According to the recent MD simulation studies, the reversible reaction between NP and ligands during the permeation, cooperative interaction induced by multiple NPs, and application of an external electric field can result in the direct permeation of NPs across a cell membrane.
Despite the recent experimental and theoretical progress on the NP-cell membrane interactions, many questions remain and many issues are still unsolved. For example, it is necessary to perform the experimental investigation and computational study under an identical system and condition. This will enable to validate the simulation results with the experimental results. New experimental systems and techniques as well as new computational methods are necessary to fill the gap between “the real system” and “the ideal system”. This will greatly contribute to the comprehensive understanding of the NP direct permeation and the development of new direct delivery technology with high delivery efficacy and less cellular damage.
This work was supported by the Hosokawa Powder Technology Foundation Research Grant. This work was also supported by the Japan Society for the Promotion of Science, KAKENHI Grant Number 1281208200 and 1581210400.
Hideya Nakamura
Dr. Hideya Nakamura is an Associate Professor at Department of Chemical Engineering in Osaka Prefecture University. He received his Ph.D. in 2008 from Osaka Prefecture University. He then worked as a Post doc associate in Particle Engineering Research Center (PERC), University of Florida. From 2010, he started his carrier as a faculty at Osaka Prefecture University. He is currently working on modelling and experimental studies on nanoparticle-cell membrane interactions. His current projects also include computational modelling of powder handling processes as well as functionalization of particulate materials for next generation secondary batteries.
Satoru Watano
Prof. Satoru Watano, specialized in Chemical Engineering, received his Ph.D. from Osaka Prefecture University (OPU) in 1995. He became professor at OPU in 2005. Currently, he is the vice dean of College of Engineering at OPU. He is the board directors of The Society of Powder Technology Japan and the council member of Japan Society of Pharmaceutical Machinery and Engineering. His research area covers (i) measurement, control, optimization of powder handling processes (ii) particle design and modification of surface properties, (iii) design and handling of nano-particles, (iv) numerical modeling and simulation of particulate systems, and (v) development of innovative powder handling processes.