Abstract
This work demonstrates the fabrication of a nanoporous iron carbide-iron oxide/reduced graphene oxide (IC-IO/ rGO) hybrid via a controlled one-step thermal treatment of Prussian blue (PB)/GO hybrid at 450 °C under N2 flow. The PB/GO hybrid is initially prepared through the in-situ deposition of PB nanoparticles on the GO sheets through electrostatic interactions. The morphological analysis of the hybrid reveals the uniform coverage of the rGO sheets by IC-IO nanoparticles and the even distribution of carbon (C), oxygen (O), and iron (Fe) on the rGO nanosheets. As a result of the hybrid composition and controlled morphology, the surface area of the obtained IC-IO/rGO hybrid (~40 m2/g) is significantly enhanced compared to those of the calcined GO sheets and PB nanoparticles (without GO).
1. Introduction
Metal-organic frameworks (MOFs) or porous coordination polymers (PCPs) have attracted significant interest as porous materials because of their high surface area, large pore volume, controllable composition and pore size, etc. (Wang Z.-L. et al., 2018; Salunkhe R. R. et al., 2016; Kaneti Y. V. et al., 2017). Prussian blue (PB) and its analogues (PBAs) exhibit several attractive characteristics, such as open framework structures, high thermal stability, and good redox activity. These CPs have been employed in numerous applications, including drug delivery (Lin W. et al., 2009), energy storage (Paolella A. et al., 2017), separation (Bureekaew S. et al., 2008), and so on (Doty F. et al., 2009; Ishizaki M. et al., 2013). Nevertheless, the poor conductivity and low chemical stability of PB presents major challenges for their practical applications (Salunkhe R. R. et al., 2015). Therefore, extensive efforts are needed to enhance the stability and conductivity of PB and its derived materials. To date, PB and PBAs have been employed as novel precursors for obtaining various transition metal compounds, including metal oxides and metal carbides (Azhar A. et al., 2019a). Generally, the calcination of PB in air produces iron oxide (IO) as a result of the removal of the cyano-group and the oxidation of the iron species. Our group previously synthesized PB and completely transformed it into nanoporous iron oxide hybrids (Azhar A. et al., 2019b), such as β-Fe2O3 (Machala L. et al., 2013), and mixed crystalline phases of iron oxide (α, γ and β-phases) (Roy X. et al., 2011). On the contrary, when pure PB was heated under inert atmosphere, the formation of metallic iron and iron carbide (IC) (Fe7C3, Fe2C, and Fe3C) was observed (Zakaria M. B. et al., 2016). Very recently, pure nickel carbide (Ni3C) was successfully prepared from cyano-bridged CPs through a thermal treatment under inert atmosphere at 450 °C (Zakaria M. B. et al., 2019). Despite some progress, the construction of PB-derived metal carbide/ carbon hybrids are still rarely reported.
Recently, carbonaceous materials, such as graphite, graphene oxide (GO), reduced graphene oxide (rGO), and carbon nanotubes (CNTs) have been widely used to enhance the functional performance of PB-based materials (Daneshvar F. et al., 2018; Azhar A. et al., 2018). The oxygen-containing functional groups on the surface of the two-dimensional (2D) GO nanosheets can serve as excellent sites for the in-situ growth of PB nanostructures (Lee T. et al., 2015). Our group has demonstrated the fabrication of several cyano-bridged CPs grown on GO nanosheets by a variety of methods (Zakaria M. B. et al., 2019). Herein, we report the synthesis of a hybrid material combining GO sheets with PB nanoparticles which can be subsequently transformed into a nanoporous IC-IO/rGO hybrid under nitrogen atmosphere at 450 °C. The obtained IC-IO/rGO hybrid has been thoroughly characterized in terms of its composition, morphology, thermal decomposition behavior, and textural properties.
2. Experimental
2.1 Chemicals
Sodium ferrocyanide(II) decahydrate (Na4[Fe(CN)6]·10H2O, ≥ 99 %) was purchased from Sigma-Aldrich (Japan). The sulfuric acid solution (H2SO4, 98 %) was obtained from Nacalai Tesque (Japan). Potassium hydroxide (KOH), sodium nitrate (NaNO3, ≥ 99 %), and ferric chloride hexahydrate (FeCl3·6H2O, ≥ 98 %) were sourced from FUJIFILM Wako Corporation (Japan). Graphite nanoplatelets (N008-100-N, thickness ~100 nm) were obtained from Angstron materials (USA). Potassium permanganate (KMnO4, ≥ 99 %) and hydrogen peroxide solution (H2O2, 30 wt.% in H2O) were purchased from Kanto Chemicals (Japan). All chemicals were utilized as received without further purification.
2.2 Preparation of GO nanosheets
The thin GO nanosheets were fabricated by employing the modified Hummer’s approach (Tanaka S. et al., 2017). In the first step, 0.33 g of graphite powder and 0.17 g of NaNO3 were mixed together and stirred. Following this, 7.67 mL of concentrated H2SO4 solution was slowly poured into this suspension and then stirred for 1 h. Next, 1.0 g of KMnO4 was added into the mixture solution which was placed in an ice bath below 20 °C. The mixture was subsequently stirred at 35 °C for 2 h and distilled water (83 mL) was added into this mixture solution under strong stirring. After that, 1.67 mL of aqueous H2O2 solution (30 % w/w) was added into the suspension. The final GO suspension was washed a number of times with a diluted HCl solution and distilled water. Finally, this GO suspension was sonicated in distilled water to exfoliate the GO sheets. The GO sheets were collected by centrifugation and subjected to repeated washing with distilled water, before being dried at ambient temperature, followed by a final drying in vacuum at 60 °C overnight. The as-prepared GO powder was dispersed in water to prepare an aqueous GO solution (2 mg mL−1) to be used in subsequent steps.
2.3 In-situ deposition of PB nanoparticles on GO nanosheets (PB/GO hybrid) and conversion to iron carbide-iron oxide/rGO hybrid
Typically, 40 mL of 0.299 mM FeCl3·6H2O solution was poured into the GO solution (20 mL, 2 mg mL−1) and stirred for 0.5 h. The mixture solution was slowly mixed with 40 mL of Na4[Fe(CN)6]·10H2O solution (0.358 mM) and stirred for further 0.5 h, before being aged for two days. The product was isolated via centrifugation and washed multiple times with water and ethanol, and finally dried at room temperature. The porous IC-IO/rGO hybrid was achieved by calcining the PB/GO hybrid at 450 °C for an hour under nitrogen (N2) flow with a ramping rate of 5 °C min−1. For comparison, pristine PB nanoparticles without GO sheets were also prepared and heated under the same conditions.
2.4 Characterization
The purity and compositions of the samples were checked by X-ray diffraction (XRD) with a Rigaku RINT 2500X diffractometer utilizing Cu Kα (1.5406 Å) radiation. Nitrogen sorption isotherms were collected using a Quantachrome Autosorb at 77 K. To dehydrate the samples, they were subjected to degassing at 250 °C for 16 h prior to the BET measurement. Morphological observations of the products were conducted using both scanning electron microscope (SEM, Hitachi SU8000) and transmission electron microscope (TEM, JEOL JEM-2100F). The infrared (IR) spectra of the samples were collected using a Thermoscientific Nicolet 4700 spectrometer (Waltham, MA, USA). Raman spectroscopy measurements were performed using a Horiba-Jovin Yvon T64000 Raman spectrometer. Thermogravimetry (TG) measurements were carried out with a Hitachi HT-Seiko Instrument Exter 6300 under N2 atmosphere from 30 to 550 °C at a ramping rate of 5 °C min−1.
3. Results and discussion
The PB/GO hybrid was obtained by the in-situ deposition of PB nanoparticles on the GO sheets (Azhar A. et al., 2019b). First, the GO sheets were synthesized by the exfoliation of graphite based on the modified Hummer’s method (Tanaka S. et al., 2017). The SEM image of the prepared GO sheets (Fig. 1a) shows a two-dimensional (2D) crumpled sheet-like structure. The zeta potential measurements reveal the change in the surface charge of the GO sheets from negative to positive charge after the modification with PB nanoparticles. The morphology of the PB/the GO hybrid is depicted in Fig. 1c, which shows the wrapping of the PB nanoparticles by the GO sheets (i.e., the surface of the GO sheets is bumpy.). The TEM image further shows that the PB nanoparticles are successfully deposited on the surface of the GO sheets (Fig. 1d). For comparison, pristine PB without GO sheets was also prepared (Fig. 1b). Fig. 2 shows the XRD pattern of the PB/GO hybrid which reveals the diffraction peaks belonging to face-centered cubic (fcc) phase of PB (JCPDS No. 73-0687) (Cao L. et al., 2010). Importantly, after the modification with PB, the primary diffraction peak belonging to the GO nanosheets disappears, possibly due to the nearly complete surface coverage of the GO sheets by the PB nanoparticles (Islam M. N. et al., 2018).

Further compositional analyses of the PB nanoparticles, GO sheets, and PB/GO hybrid were carried out using Fourier-transform infrared (FTIR) spectroscopy. The FTIR spectrum of the PB/GO hybrid (Fig. 3a) shows the presence of strong bands belonging to the cyano (CN) group of PB (Fe2+-CN-Fe3+) (Ge C.-X. et al., 2018). The peaks originating from oxygen-containing functional groups (Vermisoglou E. et al., 2014) on the GO surface is significantly reduced following the modification with PB nanoparticles, indicating the complete reduction of GO to reduced graphene oxide (rGO) (Ren S. et al., 2012) and the formation of PB on GO sheets.

The thermal degradation of PB under nitrogen flow was studied by TGA (Fig. 4). The TG curve of pure PB shows multi-step weight loss (Sun D. et al., 2019). In the first stage, lattice water and adsorbed water molecules in the structure are removed at temperatures between 30 and 210 °C. The second weight loss (210–420 °C) can be attributed to the loss of the cyano (CN) group present in the PB nanoparticles, and the final weight loss above 420 °C is correlated to the formation of IC. The thermal stability of the pure GO nanosheets under inert atmosphere was also investigated by TG measurements (Fig. 4). The evaporation of adsorbed water molecules and the removal of labile oxygen functional groups occur at ~250 °C and no further weight loss is observed (Park S. et al., 2011).
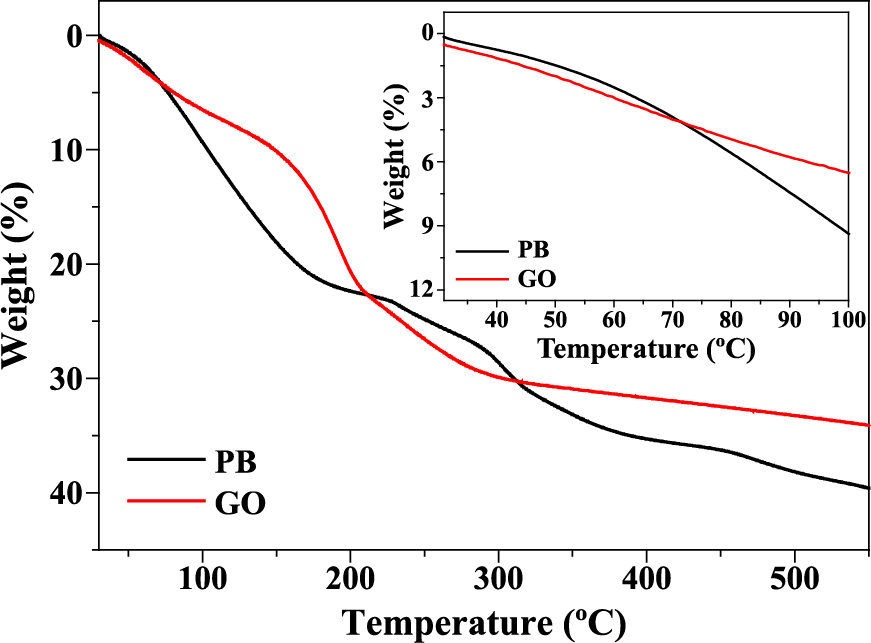
As depicted in Scheme 1, the thermal treatment of the PB/GO hybrid at 450 °C under N2 atmosphere results in the formation of the IC-IO/rGO hybrid. The crystal structure of this hybrid after calcination was examined by wide-angle XRD (Fig. 2). Some peaks are assignable to iron carbide (Fe3C, IC) (Fletcher D. et al., 2019), while the other peaks at 35.5° and 62.9° can be attributed to iron oxide (γ-Fe2O3) (Zhu K. et al., 2018), suggesting the presence of multiple iron phases after the heat treatment. Compared to the calcined PB (without GO), the relative intensity of the IO peak in the IC-IO/rGO hybrid is increased, which may be due to the reaction of iron species with oxygen functional groups of GO sheets.

Fig. 5a displays the SEM image of the IC-IO/rGO hybrid, which is composed of irregular and porous structures due to the removal of the organic group after calcination. Also, some IC-IO hybrid nanoparticles can be observed on the surface of the GO nanosheets. The TEM image clearly reveals the uniform decoration of the rGO sheets by IC-IO nanoparticles (Fig. 5b). The corresponding elemental mapping images in Fig. 5c–f confirm the uniform distribution of carbon (C), oxygen (O), and iron (Fe) on the rGO nanosheets.
The N2 sorption isotherms of the calcined PB and the IC-IO/rGO hybrid are given in Fig. 6. The specific surface area of the IC-IO/rGO hybrid (38.7 m2/g) is significantly larger than those of the calcined GO sheets (1.25 m2/g) (Zakaria M. B. et al., 2019) and calcined PB (18.2 m2/g). This is likely due to the presence of IC-IO nanoparticles which may serve as effective spacers between the GO sheets, thus preventing the stacking of the GO sheets during the thermal treatment.

The FTIR spectrum of the IC-IO/rGO hybrid (Fig. 3a) clearly shows the disappearance of many oxygen-containing functional groups, which suggests the successful reduction of GO sheets to reduced GO (rGO) (Liu H. et al., 2015). Additionally, a weak IR band belonging to the CN group is also observed. Raman spectra of the GO sheets before and after the thermal treatment are compared in Fig. 3b. The D and G bands are clearly observed. However, the positions of the D and G bands are shifted after the thermal treatment. Compared to the ID/IG value of pure GO (1.02), the ID/IG value of the IC-IO/rGO hybrid is higher (1.12), suggesting the increase of structural defects in the GO sheets after calcination.
4. Conclusions
In summary, we have successfully achieved the in-situ deposition of PB nanoparticles on the surface of GO sheets through the interaction of PB with the oxygen-containing functional groups of GO nanosheets. This PB/GO hybrid can be converted to the IC-IO/rGO hybrid via a one-step thermal treatment at 450 °C under N2 flow with well-retained morphology. This finding indicates that the presence of rGO during thermal treatment helps to promote the formation of iron carbide at high temperatures. The surface area of the obtained IC-IO/rGO hybrid (~40 m2/g) is superior to those of the calcined GO sheets and the calcined PB without GO. This hybrid material composed of multiple compositions may have many potential applications in supercapacitors and oxygen reduction reaction (ORR).
Acknowledgements
This work was conducted at the Queensland node of the Australian National Fabrication Facility (ANFF-Q), a company established under the National Collaborative Research Infrastructure Strategy to provide nano and microfabrication facilities for Australian researchers. S.M.A., T.A., and Y.Y. thank Researchers Supporting Project Number (RSP-2020/6), King Saud University, Riyadh, Saudi Arabia.
References
- Azhar A., Li Y., Cai Z., Zakaria M.B., Masud M.K., Hossain M.S.A., Kim J., Zhang W., Na J., Yamauchi Y., Hu M., Nanoarchitectonics: a new materials horizon for Prussian blue and its analogues, Bulletin of the Chemical Society of Japan, 92 (2019a) 875–904. DOI: 10.1246/bcsj.20180368
- Azhar A., Yamauchi Y., Allah A.E., Alothman Z.A., Badjah A.Y., Naushad M., Habila M., Wabaidur S., Wang J., Zakaria M.B., Nanoporous iron oxide/carbon composites through in-situ deposition of Prussian blue nanoparticles on graphene oxide nanosheets and subsequent thermal treatment for supercapacitor applications, Nanomaterials, 9 (2019b) 776. DOI: 10.3390/nano9050776
- Azhar A., Zakaria M.B., Lin J., Chikyow T., Martin D.J., Alghamdi Y.G., Alshehri A.A., Bando Y., Hossain M.S.A., Wu K.C.-W., Kumar N.A., Yamauchi Y., Graphene-wrapped nanoporous nickel-cobalt oxide flakes for electrochemical supercapacitors, ChemistrySelect, 3 (2018) 8505–8510. DOI: 10.1002/slct.201801174
- Bureekaew S., Shimomura S., Kitagawa S., Chemistry and application of flexible porous coordination polymers, Science and Technology of Advanced Materials, 9 (2008) 014108. DOI: 10.1088/1468-6996/9/1/014108
- Cao L., Liu Y., Zhang B., Lu L., In situ controllable growth of Prussian blue nanocubes on reduced graphene oxide: facile synthesis and their application as enhanced nanoelectrocatalyst for H2O2 reduction, ACS Applied Materials & Interfaces, 2 (2010) 2339–2346. DOI: 10.1021/am100372m
- Daneshvar F., Aziz A., Abdelkader A.M., Zhang T., Sue H.-J., Welland M.E., Porous SnO2–Cux O nanocomposite thin film on carbon nanotubes as electrodes for high performance supercapacitors, Nanotechnology, 30 (2018) 015401. DOI: 10.1088/1361-6528/aae5c6
- Doty F.P., Bauer C.A., Skulan A.J., Grant P.G., Allendorf M.D., Scintillating metal-organic frameworks: a new class of radiation detection materials, Advanced Materials, 21 (2009) 95–101. DOI: 10.1002/adma.200801753
- Fletcher D.C., Hunter R., Xia W., Smales G.J., Pauw B.R., Blackburn E., Kulak A., Xin H., Schnepp Z., Scalable synthesis of dispersible iron carbide (Fe3C) nanoparticles by ‘nanocasting’, Journal of Materials Chemistry A, 7 (2019) 19506–19512. DOI: 10.1039/C9TA06876G
- Ge C.-X., Li P.-J., Lai J.-H., Qiu P., In situ synthesis and characterization of Prussian blue nanocubes on graphene oxide and its application for H2O2 reduction, Indian Journal of Chemistry, 57A (2018) 26–33. http://nopr.niscair.res.in/handle/123456789/43488
- Ishizaki M., Tsuruta S., Kanaizuka K., Sakamoto M., Kawamoto T., Tanaka H., Kurihara M., Growth of Pt subnano clusters on limited surface areas of Prussian blue nanoparticles, Journal of Inorganic and Organometallic Polymers and Materials, 23 (2013) 216–222. DOI: 10.1007/s10904-012-9721-9
- Islam M.N., Gorgannezhad L., Masud M.K., Tanaka S., Hossain M.S.A., Yamauchi Y., Nguyen N.-T., Shiddiky M.J.A., Graphene-oxide-loaded superparamagnetic iron oxide nanoparticles for ultrasensitive electrocatalytic detection of microRNA, ChemElectroChem, 5 (2018) 2488–2495. DOI: 10.1002/celc.201800339
- Kaneti Y.V., Tang J., Salunkhe R.R., Jiang X., Yu A., Wu K.C.-W., Yamauchi Y., Nanoarchitectured design of porous materials and nanocomposites from metal-organic frameworks, Advanced Materials, 29 (2017) 1604898. DOI: 10.1002/adma.201604898
- Lee T., Min S.H., Gu M., Jung Y.K., Lee W., Lee J.U., Seong D.G., Kim B.-S., Layer-by-layer assembly for graphene-based multilayer nanocomposites: synthesis and applications, Chemistry of Materials, 27 (2015) 3785–3796. DOI: 10.1021/acs.chemmater.5b00491
- Lin W., Rieter W.J., Taylor K.M.L., Modular synthesis of functional nanoscale coordination polymers, Angewandte Chemie International Edition, 48 (2009) 650–658. DOI: 10.1002/anie.200803387
- Liu H.D., Zhang J.L., Xu D.D., Huang L.H., Tan S.Z., Mai W.J., Easy one-step hydrothermal synthesis of nitrogen-doped reduced graphene oxide/iron oxide hybrid as efficient supercapacitor material, Journal of Solid State Electrochemistry, 19 (2015) 135–144. DOI: 10.1007/s10008-014-2580-2
- Machala L., Zoppellaro G., Tuček J., Šafářová K., Marušák Z., Filip J., Pechoušek J., Zbořil R., Thermal decomposition of Prussian blue microcrystals and nanocrystals – iron(iii) oxide polymorphism control through reactant particle size, RSC Advances, 3 (2013) 19591–19599. DOI: 10.1039/C3RA42233J
- Paolella A., Faure C., Timoshevskii V., Marras S., Bertoni G., Guerfi A., Vijh A., Armand M., Zaghib K., A review on hexacyanoferrate-based materials for energy storage and smart windows: challenges and perspectives, Journal of Materials Chemistry A, 5 (2017) 18919–18932. DOI: 10.1039/C7TA05121B
- Park S., An J., Potts J.R., Velamakanni A., Murali S., Ruoff R.S., Hydrazine-reduction of graphite- and graphene oxide, Carbon, 49 (2011) 3019–3023. DOI: 10.1016/j.carbon.2011.02.071
- Ren S., Li R., Meng X., Li H., Self-assembly of reduced graphene oxide at liquid–air interface for organic field-effect transistors, Journal of Materials Chemistry, 22 (2012) 6171–6175. DOI: 10.1039/C2JM16232F
- Roy X., Hui J.K.-H., Rabnawaz M., Liu G., MacLachlan M.J., Soluble Prussian blue nanoworms from the assembly of metal–organic block ionomers, Angewandte Chemie International Edition, 50 (2011) 1597–1602. DOI: 10.1002/anie.201005537
- Salunkhe R.R., Kaneti Y.V., Kim J., Kim J.H., Yamauchi Y., Nanoarchitectures for metal–organic framework-derived nanoporous carbons toward supercapacitor applications, Accounts of Chemical Research, 49 (2016) 2796–2806. DOI: 10.1021/acs.accounts.6b00460
- Salunkhe R.R., Tang J., Kamachi Y., Nakato T., Kim J.H., Yamauchi Y., Asymmetric supercapacitors using 3D nanoporous carbon and cobalt oxide electrodes synthesized from a single metal–organic framework, ACS Nano, 9 (2015) 6288–6296. DOI: 10.1021/acsnano.5b01790
- Sun D., Wang H., Deng B., Zhang H., Wang L., Wan Q., Yan X., Qu M., A Mn-Fe based Prussian blue Analogue@Reduced graphene oxide composite as high capacity and superior rate capability anode for lithium-ion batteries, Carbon, 143 (2019) 706–713. DOI: 10.1016/j.carbon.2018.11.078
- Tanaka S., Salunkhe R.R., Kaneti Y.V., Malgras V., Alshehri S.M., Ahamad T., Zakaria M.B., Dou S.X., Yamauchi Y., Hossain M.S.A., Prussian blue derived iron oxide nanoparticles wrapped in graphene oxide sheets for electrochemical supercapacitors, RSC Advances, 7 (2017) 33994–33999. DOI: 10.1039/C7RA03179C
- Vermisoglou E.C., Devlin E., Giannakopoulou T., Romanos G., Boukos N., Psycharis V., Lei C., Lekakou C., Petridis D., Trapalis C., Reduced graphene oxide/iron carbide nanocomposites for magnetic and supercapacitor applications, Journal of Alloys and Compounds, 590 (2014) 102–109. DOI: 10.1016/j.jallcom.2013.11.087
- Wang Z.-L., Sun K., Henzie J., Hao X., Ide Y., Takei T., Bando Y., Yamauchi Y., Electrochemically in situ controllable assembly of hierarchically-ordered and integrated inorganic–carbon hybrids for efficient hydrogen evolution, Materials Horizons, 5 (2018) 1194–1203. DOI: 10.1039/C8MH00773J
- Zakaria M.B., Nanostructuring of nanoporous iron carbide spheres via thermal degradation of triple-shelled Prussian blue hollow spheres for oxygen reduction reaction, RSC Advances, 6 (2016) 10341–10351. DOI: 10.1039/C5RA24357B
- Zakaria M.B., Tan H., Kim J., Badjah A.Y., Naushad M., Habila M., Wabaidur S., Alothman Z.A., Yamauchi Y., Lin J., Structurally controlled layered Ni3C/graphene hybrids using cyano-bridged coordination polymers, Electrochemistry Communications, 100 (2019) 74–80. DOI: 10.1016/j.elecom.2019.01.004
- Zhu K., Chen C., Xu M., Chen K., Tan X., Wakeel M., Alharbi N.S., In situ carbothermal reduction synthesis of Fe nanocrystals embedded into N-doped carbon nanospheres for highly efficient U(VI) adsorption and reduction, Chemical Engineering Journal, 331 (2018) 395–405. DOI: 10.1016/j.cej.2017.08.126
Authors’ Short Biographies
Alowasheeir Azhar

Dr. Alowasheeir Azhar received her BSc in Energy Engineering (2013), MSc in Applied Science and Chemistry (2015) from Tokai University (Japan), and Ph.D. (2019) in Department of Nanoscience and Nanoengineering at Waseda University (Japan). She received the Custodian of The Two Holy Mosques’ overseas scholarship program (2007 to 2019) from Kingdom of Saudi Arabia. Her current research interest is the design for hybrid materials and nanoporous materials.
Jacob Earnshaw

Jacob Earnshaw received his BSc (Chemistry) degree from the School of Chemistry and Molecular Biosciences at the University of Queensland, Australia in 2018. Currently, he is an Honours student under the supervision of Professor Yusuke Yamauchi, and his research pursues the synthesis of nanoarchitectured materials for energy storage and electrocatalysis.
Mohamed B. Zakaria

Dr. Mohamed B. Zakaria is a senior researcher at Australian Institute for Bioengineering and Nanotechnology (AIBN), the University of Queensland, Australia and a Lecturer of Physical Chemistry, Department of Chemistry, Faculty of Science, Tanta University, Egypt. He received his BSc in Chemistry and MSc in Physical Chemistry from Tanta University. Then, he received his PhD in Engineering in 2016 from Waseda University, Japan. He did his postdoctoral research at NIMS-MANA, Tsukuba, Japan as a JSPS Fellow from 2016–2018. His area of interest is in the fields of Chemistry, Materials Design, Hybrid Materials, Energy, Catalysis, Dielectric Capacitors, Ferroelectric Materials, and Applied Nanomaterials.
Ping Cheng

Ping Cheng received a MSc in biotechnology in 2019 at the University of Queensland. He is currently studying for Ph.D. in the group of Prof. Yusuke Yamauchi, Australian Institute for Bioengineering and Nanotechnology. His research interests include environmental applications and biological applications of porous materials.
Yusuf Valentino Kaneti

Dr. Yusuf Valentino Kaneti received his PhD from the University of New South Wales in July 2014 under the supervision of Prof. Aibing Yu. He joined the group of Prof. Yusuke Yamauchi at the National Institute of Materials Science as a JSPS Postdoctoral Fellow in September 2016. He is now working as a MANA Research Associate at NIMS. His research interest is focused on the fabrication of functional porous materials for energy and environmental applications.
Md. Shahriar A. Hossain

Dr. Md Shahriar A. Hossain is currently a Senior Lecturer in the School of Mechanical and Mining Engineering at UQ. He obtained his Ph.D degree in Materials Science and Engineering, The University of Wollongong (UoW), Australia in 2008. He was then employed as a Postdoctoral Research Fellow at the University of Geneva, Switzerland. He was awarded the prestigious DECRA Fellowship from the Australian Research Council (ARC) in 2013. His research experience in various institutes and in industry around the world has been mainly focused on fabrication and characterization of superconducting materials and magnetically triggered nanoparticles for medical and energy applications.
Saad M. Alshehri

Prof. Saad M Alshehri received his Ph.D. in Inorganic Chemistry from the Leicester University, England in 1992. He worked at Northeastern University, Boston and the Leicester University as a post-doctoral fellow. Prior to join to King Saud University, Prof. Saad was an Assistant Professor of Chemistry at King Khalid Military Academy, Riyadh, Saudi Arabia. His research interests are in synthesis of inorganic materials and their industrial applications. He has synthesized heat resistant and thermally stable material. He has also investigated inorganic and hybrid materials for electro-catalyst and for energy storage devices such as electrodes for lithium ions batteries and supercapacitors.
Tansir Ahamad

Dr. Tansir Ahamad received his PhD degree in 2006 from Jamia Millia Islamia, New Delhi (INDIA). After his graduation, he was employed as a post-doc at the University of Western Cape (South Africa). At the conclusion of his post-doc position he joined King Saud University, Saudi Arab in 2008 and was employed as an Assistant Professor. From March 2020, he has started his academic employment as a full professor in the department of Chemistry at King Saud University. His major research interest is in the fabrication of porous nanomaterials, organic-inorganic hybrid materials, and their applications in energy and environments.
Yusuke Yamauchi

Prof. Yusuke Yamauchi received his bachelor’s degree (2003), master’s degree (2004), and PhD degree (2007) from Waseda University, Japan. After that, he joined the National Institute for Materials Science to start his research group. Currently, he is a full professor at the School of Chemical Engineering and a senior group leader at AIBN in The University of Queensland. He concurrently serves as an associate editor of J Mater Chem A (RSC) and Chem Eng J (Elsevier). He has published over 750 papers with >36,000 citations (h-index 97, Web of Science®). He was selected as the Highly Cited Researchers in Chemistry in 2016–2019.
Jongbeom Na

Dr. Jongbeom Na received his Ph.D. degree (2017) from the Department of Chemical and Biomolecular Engineering at Yonsei University (Republic of Korea). Until 2018, he worked as a Research Engineer at Chemical Laboratory of SK Chemicals Co., Ltd. Currently, he is working as a research fellow at AIBN, the University of Queensland and MANA, NIMS. His major research interest is in the design and synthesis of functional nanomaterials, organic-inorganic hybrid materials, and their applications.


















