2014 Volume 3 Issue Special_Issue Pages S0027
2014 Volume 3 Issue Special_Issue Pages S0027
The development of a MALDI-TOF mass spectrometer that utilizes spiral ion trajectory, SpiralTOF, is reported. The total flight path length was 17 m, which is five times longer than that in commonly used reflectron ion optical system. The SpiralTOF reduced the dependence of the mass resolving power on the mass of the analyte, while improving the accuracy of the mass measurements. Furthermore, SpiralTOF has two advantages that can be exploited for the separation of minor abundant isobaric components in mass spectra. One is the reduction in chemical background due to the post source decay (PSD), which is achieved through PSD ion elimination by electrostatic sectors contained within the SpiralTOF. The other is that the stabilities of peak positions are improved during mass spectrum accumulation. The peak drift caused by the fine structure of matrix crystals and the small irregularities on the sample surface can be reduced by extending the flight path. In this study, these advantages are demonstrated via the analysis of a block copolymer and mass spectrometry imaging (MSI) of lipids.
The invention of the delayed extraction technique1,2) has resulted in a dramatic improvement in matrix-assisted laser desorption/ionization (MALDI)3,4)-time-of-flight (TOF)5,6) mass spectrometry. However, the mass range that can be effectively evaluated is relatively narrow because of an intrinsic feature of the ionization mechanism. In MALDI, ions and neutral particles gain similar velocities in the plume.7) The average velocity is weakly dependent on mass value, with the initial kinetic energy increasing in proportion to mass value, and this leads to the mass being dependent on the location of the focal point. This dependence in turn results in the mass resolution being dependent on the mass itself and reduces mass accuracy. In order to improve the mass resolution of ions over a wide mass range, it is necessary to reduce the mass dependence of the delayed extraction technique. The ion optical system of a MALDI-TOF mass spectrometer is comprised of two systems that are connected in tandem. The first system focuses the initial kinetic energy distribution using the delayed extraction technique at a particular focal point, while the second focuses the kinetic distribution of the focal point on the detection surface. To achieve good mass resolution over a wide mass range, the total flight path length needs to be extended, and the contribution of the first system should be reduced. We have adopted an ion optical system that employs a spiral ion trajectory8–10) (SpiralTOF) rather than the more commonly used ion mirror. This system is based on a multi-turn ion optical system that uses electrostatic sectors,11–16) in particular the “MULTUM II” system developed at Osaka University, Japan.15,16) In a previous report we discussed the basic performance of this system10) and confirmed that a superior mass resolution and mass accuracy could be successfully achieved over a wide mass range.
The focus of the present study was on the separation of minor isobaric components, which encompasses a large quantity of the information that is obtained from mass spectra. There are two additional requirements for analyzing minor components such as these. One is the reduction in chemical background due to post source decay (PSD), which can be achieved by the elimination of PSD ions by electrostatic sectors contained within the SpiralTOF. The other is improvement of the stabilities of peak positions during mass spectrum accumulation. The fine structure of the matrix crystals and small irregularities in the sample surface can cause a peak drift in mass spectra, which is caused by slight differences in the starting point of the flight path for the ions at each laser irradiation point. SpiralTOF, the flight path of which is 5–10 times longer than the reflectron-type TOF, is able to reduce the effect of this mass drift, which results in achieving high mass resolution and high mass accuracy. In this article, we report the exploitation of these advantages for the analysis of block copolymers, in addition to the mass spectrometry imaging (MSI) of lipids, both of which require the separation of isobaric compounds for a detailed analysis to be achieved.
Poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (average MW 1100 (EO–PO block copolymer)) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The matrix compound, α-cyano-4-hydroxycinnamic acid (HCCA), acetonitrile (ACN), and trifluoroacetic acid (TFA) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). HCCA was dissolved in a mixture of ACN/0.1% TFA in deionized water (1 : 1) at a concentration of 10 mg/mL. The EO–PO block copolymer and sodium iodide were prepared by dilution in deionized water at concentrations of 10 mg/μL and 1 mg/μL, respectively. The EO–PO block copolymer, HCCA, and sodium iodide solutions were mixed in a ratio of 1 : 2 : 1, and 0.5 μL of the mixture was deposited on the MALDI target plate using the dried-droplet method.
MSI analysisThis study was approved by the Institutional Animal Experiments Committee and conducted in accordance with the guidelines of animal experimentation at Osaka University. Six-week-old male C57BL/6J mice were anesthetized with isoflurane (Mylan Inc., PA, USA), sacrificed, and dissected. The brain tissue was immediately frozen in powdered dry ice to minimize degradation and was then maintained at −80°C. The tissue was sectioned using a cryostat to produce 10 μm thick slices, which were then thaw-mounted on an indium–tin–oxide glass sample plate (HST Inc., NJ, USA). The samples were dehydrated in a vacuum chamber and the plate was mounted on a sample holder. The matrix compound, 2,5-dihydroxybenzoic acid (DHB) (Sigma-Aldrich, MO, USA) was dissolved in 100% methanol at a concentration of 30 mg/mL. A total volume of 2 mL of matrix solution was sprayed onto the individual tissue sections.
The SpiralTOF (JMS-S3000, JEOL Ltd., Akishima, Japan) containing the spiral ion optical system and comprised of four toroidal electrostatic sectors was used for the measurements.10) A schematic of the system is shown in Fig. 1. The red line represents the ion trajectory passing through an eight-cycle SpiralTOF to the detector. A few improvements to this system have been made since the publication of its description in ref. 10. A solid-state Nd:YLF laser with a wavelength of 349 nm (model: Explorer349, Newport, CA, USA), was used for ionization. The laser spot was controlled to 20 μm and the laser fluence was 30 μJ. A secondary electron multiplier ion detector, DM291 (ETP, Ermington, Australia), and an 8-bit digitizer, U1082A (Agilent, CA, USA), were used for ion detection and data acquisition, respectively.

The delayed extraction method was employed in the experiments to optimize mass resolving power. The delay time was adjusted so as to obtain the maximum resolving power at m/z 1,000 and m/z 800 for the co-polymer analysis and MSI measurement, respectively. The MSI measurements were performed on the left half of the brain tissue sections (5 mm×7 mm) with a spatial resolution of 40 μm. The mass spectrum in each pixel was acquired using 2,000 laser shots with a 1-kHz laser repetition rate. Because of the laser irradiation diameter, 20 μm, was smaller than a pixel size, a laser irradiation point was randomly changed within a pixel region in each 500 laser shots for 4 times.
The mass spectrum of the EO–PO block copolymer is shown in Fig. 2. The observed peaks are sodium adducted ions, [M+Na]+, of different combinations of EO and PO units. The EO and PO components are expressed (number of EO units, number of PO units) below. In the detailed analysis using high-resolution SpiralTOF, many doublet peaks due to different combinations of EO and PO units were observed. As an example, an enlarged mass spectrum at m/z 1027.2–1033.2 is shown in Fig. 3. The solid arrows indicate an isotopic pattern of (0, 17), and the dotted arrows point to that of (4, 14). The doublet peaks observed at 1029.7, with a 0.03 u difference, are enlarged. The third peak of (0, 17) and the monoisotopic ion peak of (4, 14) can be seen to be clearly resolved owing to the high mass resolving power of 80,000 for this system. In contrast, no (4, 14) peak was found using a reflectron-type MALDI-TOF mass spectrometer. Furthermore, one of the advantages of extending the flight path length is that highly resolved peaks still contain adequate points. This would be expected to enhance the reliability of peak area analysis, which is related to relative ion abundance, and is important for estimating the molecular structure of copolymers.17)


The averaged mass spectrum of all image pixels is shown in Fig. 4. The base peak ion at m/z 798 was estimated as arising from phosphatidylcholine (PC) (34 : 1) [M+K]+. The mass image constructed for m/z 798 with a mass window of ±0.1 u, is also shown in Fig. 4. This image shows that the PC (34 : 1) is distributed uniformly throughout the brain tissue section. Nine regions-of-interest (ROI), 1–9, were selected to allow peak drift to be evaluated. The m/z variation of PC (34 : 1) [M+K]+ at ROI 1–9 shown in Fig. 5, was within ±10 ppm. The overlaid peak shapes of the PC (34 : 1) [M+K]+ in the accumulated mass spectra of ROI 1, 4, and 5 are shown in Fig. 6. The mass drift is caused by small irregularities in the sample surface. By extending the flight path and reducing the affect to a reasonably low level during the MSI measurements, it was considered to be sufficient for constructing a high resolution mass image.



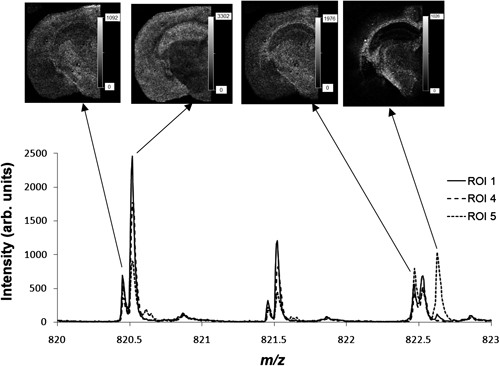
It is important to note that, for the mass analysis of lipids in mouse brain tissue, various types of lipid ions are observed as minor components in the mass spectra. For example, the accumulated mass spectra of ROI 1, 4, and 5 at m/z 820–823 are shown in Fig. 7, with the mass differences for doublet or triplet peaks being only 0.1–0.2 u. Four types of lipids were observed within 3 u, with the m/z of their monoisotopic ions being 820.461, 820.525, 822.478, and 822.631. These were assigned to phosphatidylethanolamine (PE) (36 : 2) [M+2K−H]+ (m/z 820.466), PC (36 : 4) [M+K]+ (m/z 820.525), PE (36 : 1) [M+2K−H]+ (m/z 822.481), and galactosylceramide (GalCer) (d18:1/22 : 0) [M+K]+ (m/z 822.622) based on accurate mass analysis. The high resolution mass images of these four lipids are shown in Fig. 7, showing that they are unevenly distributed throughout the brain tissue. It is clear that if the doublet and triplet peaks were not mass resolved using a reflectron-type MALDI-TOF mass spectrometer, the inherent distribution of the compounds would be lost.18)
The advantages of extending the flight path in mass spectrometry by adopting a spiral ion trajectory consisting of electrostatic sectors are discussed and evaluated. By combining this system with MALDI, it represents a powerful tool, not only for the improvement of mass resolution over a wide mass range, but also for the separation of minor abundance isobaric components. The focus of this study was on the separation of compounds in a complex mixture, but it would be expected to be useful for the analysis of low molecular weight compounds in the presence of an interfering chemical background components. In conclusion, the spiralTOF has great potential for expanding the fields of application MALDI-TOF MS to relatively low molecular weight and low abundance ions, which is known to be difficult when a reflectron-type MALDI-TOF mass spectrometer is used.