2016 Volume 57 Issue 10 Pages 1766-1770
2016 Volume 57 Issue 10 Pages 1766-1770
A polyacrylamide gel route was adopted to synthesize Bi4Ti3O12 nanoparticles and the effect of chelating agents on the products was investigated. When citric acid and acetic acid is separately used as the chelating agent, single-phase Bi4Ti3O12 samples can be prepared at a calcination temperature of 500℃; however, when tartaric acid or EDTA is used as the chelating agent, a higher calcination temperature of 600℃ is required to produce single-phase samples. SEM observation shows that the as-prepared samples are composed of sphere-like or ellipsoid-like particles. The average particle size of the citric acid-, acetic acid-, tartaric acid-, and EDTA-resulted samples is centered around 32, 35, 90 and 120 nm, respectively. The bandgap energy of the four samples is measured to be 3.27 eV by ultraviolet-visible diffuse reflectance spectroscopy. The photocatalytic activity of Bi4Ti3O12 samples was evaluated by degrading RhB under simulated-sunlight irradiation, and the influence of pH value on the adsorption and photocatalytic degradation of the dye was also investigated. Ethanol, BQ and AO were respectively used as the scavengers of •OH, •O2− and h+ to investigate their effect on the photocatalytic degradation with the aim of revealing the photocatalytic mechanism involved. Based on the experimental results, the direct oxidation by h+ is suggested to be the main mechanism toward the dye degradation.
With the ever-increasing demand for textile, paint and cosmetic products, a proportional increase in generation of wastewater containing various organic dyes has been observed simultaneously. It is estimated that approximately one million tons of organic dyes are produced annually all over the world, most of which have complicated polyaromatic structures and are hardly decomposed by self-purification action. The organic dyes in wastewater must be removed or destroyed into harmless inorganic substances before the dye-containing effluent is released into the natural environment. Various physical and chemical methods such as adsorption, coagulation/flocculation, membrane filtration, electrolysis and advanced oxidation have been developed to decolorize the dye wastewater.1–4) However, most of them are disadvantaged by high operational cost, excessive chemical demands and strict operational conditions, which restrict their practical application in the wastewater decolourisation. In recent years, semiconductor-based photocatalysis has received a great interest because it allows the use of solar energy for the destruction of dye pollutants and is considered as a green technology for the wastewater treatment.
Titanium-based oxide semiconductors are known to be an important class of photocatalysts and exhibit powerful capabilities for photocatalytically decomposing various organic dyes into harmless inorganic substances. As one of the representatives of titanium-based semiconductors, bismuth titanate (Bi4Ti3O12) is particularly interesting. This titanium-based semiconductor has a special layer structure constructed by alternative stacking of perovskite-like (Bi2Ti3O10)2− blocks and (Bi2O2)2+ units along the c-axis orientation.5) The conduction band of Bi4Ti3O12 is mainly composed of Ti 3d + Bi 6p orbitals and the valence band consists of O 2p + Bi 6s hybrid orbitals.6) Due to its special layered crystal structure and electronic band structure, Bi4Ti3O12 possesses unique physicochemical properties such as ferroelectricity, piezoelectricity, photoelectricity and photocatalytic activity.6–10) In particular, since the discovery of the photocatalytic H2 evolution in the methanol aqueous solution and photocatalytic degradation of methyl orange over Bi4Ti3O12,6,11) Bi4Ti3O12 has been extensively studied as a promising photocatalyst.10,12–21)
The photocatalytic process is initiated by the irradiation of a semiconductor photocatalyst and the excitation of electrons from the valence band to the conduction band. The photogenerated electrons (e−) and holes (h+) migrate to the semiconductor surface and participate in redox reactions, thus leading to the decomposition of organic dyes. It is expected that the size and morphology of the photocatalyst have an important effect on its photocatalytic activity. Especially at the nanoscale the photogenerated electrons and holes can migrate to the photocatalyst surface more easily and furthermore nanostructures can provide more available surface active sites for the photocatalytic reaction. As a result, small-sized photocatalyst is expected to exhibit an enhanced photocatalytic activity. Various nano/micro-fabrication techniques have been widely used to fabricate Bi4Ti3O12 nano/micro structures such as liquid chemical reaction,10) hydrothermal process,11,12) oxidant peroxide method,13) solid-state reaction,14) aqueous sol-gel method,15) molten salt method,16,17) electrospinning process,18,19) sol–gel hydrothermal process,20) sonochemical method,21) Based on these methods, several promising morphologies such as hierarchical nanosheets, microplatelets, nanobelts and nanofibers have been prepared.12,14,16,17,19,20) However, further synthesis of small-sized Bi4Ti3O12 nanoparticles via an alternative method is still indispensable. In this study we adopted a polyacrylamide gel route as described in the literature22) to synthesize Bi4Ti3O12 nanoparticles and the effect of chelating agents on the products was investigated. The photocatalytic activity of prepared samples was evaluated by degrading rhodamine B (RhB) under simulated-sunlight irradiation. The photocatalytic mechanism involved was discussed.
All raw materials and chemical reagents are of analytical grade and used without further purification. According to the formula Bi4Ti3O12, bismuth nitrate pentahydrate (Bi(NO3)3·5H2O) and tetrabutyl titanate (C16H36O4Ti) were weighed in a mole ratio of 4:3 (the total Bi/Ti content: 0.015 mol) and dissolved in 20 mL of dilute nitric acid solution and 25 mL of ethanol, respectively. The above two solutions were mixed together. Then to the mixture solution were successively added 0.0225 mol of the chelating agent (citric acid, acetic acid, tartaric acid or ethylene diamine tetraacetic acid (EDTA)), 20 g of glucose, and 0.135 mol of acrylamide. Every step mentioned above was accompanied by a constant magnetic stirring to make the additives dissolve fully. The resultant solution was heated at 80℃ on a hot plate to initiate the polymerization reaction. The obtained gel was dried at 120℃ for 24 h in a thermostat drier. The formed xerogel was ground into powder and submitted to calcination at 500 or 600℃ for 10 h, finally yielding Bi4Ti3O12 nanoparticles.
The phase purity of the as-prepared Bi4Ti3O12 samples was checked by means of X-ray powder diffraction (XRD) with Cu Kα radiation (λ = 0.15406 nm). The particle morphology and size of the samples was investigated by a field-emission scanning electron microscope (SEM). The UV–visible diffuse reflectance spectrum (DRS) of the samples was measured on a UV–visible spectrophotometer with an integrating sphere attachment by using BaSO4 as the reference.
The photocatalytic activity of the samples was evaluated by degrading RhB in aqueous solution under simulated-sunlight irradiation from a 200 W xenon lamp (solar simulator). In all the photocatalytic experiments, the initial concentration of RhB was 5 mg L−1 and the photocatalyst loading was 0.5 g in 100 mL of RhB solution. Before illumination, the mixed suspension was magnetically stirred for 20 min in the dark in order to attain the adsorption–desorption equilibrium between RhB and photocatalyst. During the photocatalysis process, the water-jacketed reactor was cooled with a water-cooling system to keep the solution at room temperature. A small amount of the reaction solution was pipetted out and centrifuged at 4000 r min−1 for 10 min to remove the photocatalyst particles. The RhB concentration was determined by measuring the absorbance of the solution at a fixed wavelength of λ = 554 nm using a UV–visible spectrophotometer. The degradation efficiency is defined as (C0 − Ct)/C0 × 100%, where C0 and Ct represent the initial RhB concentration and the remaining RhB concentration after photocatalysis for time t, respectively. The influence of pH value on the adsorption and photocatalytic degradation of the dye over Bi4Ti3O12 particles was also investigated, where the pH was adjusted by HCl and NaOH solutions.
Photoluminescence (PL) technique was used to examine whether there were hydroxyl (•OH) radicals formed over the simulated-sunlight-irradiated Bi4Ti3O12 catalyst. In order to achieve this goal, terephthalic acid (TPA) was used as a •OH radical scavenger. TPA tends to react with •OH radicals to produce 2-hydroxyterephthalic acid (TAOH) that is a highly fluorescent compound exhibiting characteristic photoluminescence at around 429 nm. TPA was dissolved in NaOH solution (1.0 mmol∙L−1) to make a 0.25 mmol∙L−1 TPA solution. 0.5 g of the Bi4Ti3O12 catalyst was added to 100 mL of the TPA solution. After magnetically stirred for 20 min in the dark, the mixed solution was irradiated by a 200 W xenon lamp. A small amount of the reaction solution was pipetted out and centrifuged at 4000 r∙min−1 for 10 min to remove the catalyst. The reaction solution was measured at a fluorescence spectrophotometer with the excitation wavelength of 315 nm.
Figures 1(a)–(d) show the XRD patterns of Bi4Ti3O12 samples prepared by using citric acid, acetic acid, tartaric acid and EDTA as the chelating agent, respectively. The standard XRD line pattern for Bi4Ti3O12 orthorhombic structure (PDF card No. 035-0795) is also given in Fig. 1. It is seen that when citric acid and acetic acid is separately used as the chelating agent, the resulted samples crystallize in a pure Bi4Ti3O12 phase at a calcination temperature of 500℃. When tartaric acid or EDTA is used as the chelating agent, the samples prepared at 500℃ contains small amount of impurity phases of Bi7.68Ti0.32O12.16 and Bi1.74Ti2O6.624 besides the major phase of Bi4Ti3O12 orthorhombic structure. The content of the impurity phases is reduced to a negligible level by increasing the calcination temperature up to 600℃.
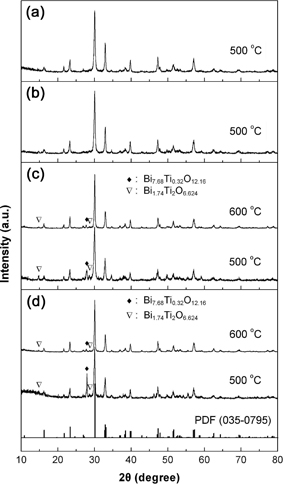
XRD patterns of Bi4Ti3O12 samples prepared by using different kinds of chelating agents, along with the standard XRD line pattern for Bi4Ti3O12 orthorhombic structure (PDF card No. 035-0795).
Figure 2 shows the SEM images of Bi4Ti3O12 samples prepared by using different chelating agents, where S1 and S2 correspond to the samples prepared at 500℃ by using citric acid and acetic acid as the chelating agent, respectively, and S3 and S4 correspond to the samples prepared at 600℃ by using tartaric acid and EDTA as the chelating agent, respectively. It is seen that all the samples are composed of sphere-like or ellipsoid-like particles, but they have different particle sizes. The major particle size distribution range (measured by Heywood diameter) of samples S1–S4 is 25–45, 30–55, 70–120 and 90–190 nm, respectively, and their average particle size is centered around 32, 35, 90 and 120 nm, respectively.

SEM images of the as-prepared single-phase Bi4Ti3O12 samples. S1 → citric acid (500℃), S2 → acetic acid (500℃), S3 → tartaric acid (600℃), S4 → EDTA (600℃).
Figure 3 shows the UV-visible diffuse reflectance spectra of Bi4Ti3O12 samples and the corresponding first derivative spectra. The peak wavelength in the first derivative spectra is characterized to be the absorption edge of the samples. It is seen that all the samples have a similar absorption edge at around 379 nm and this absorption edge is attributed to the electron transition from valence band (VB) to conduction band (CB), from which the bandgap energy Eg of the samples is obtained to be 3.27 eV. This value is slightly larger than that of Bi4Ti3O12 nanobelts (3.0 eV) prepared by solid-state reaction.14)

UV-visible diffuse reflectance spectra of Bi4Ti3O12 samples and the corresponding first derivative spectra.
Since the presence of various pH values in real industrial dye wastewater, the influence of pH on the adsorption and photocatalytic degradation of the dye is an important consideration bearing in mind. Figure 4(a) shows the time-dependent adsorption of RhB onto Bi4Ti3O12 catalyst (sample S1) under different pH values in the dark. In every case of the pH value, the dye adsorption reaches equilibrium at 10–20 min of contact time. The adsorption percentage of the dye after 20 min of adsorption is shown in Fig. 4(b). At low pH values below 3.8, Bi4Ti3O12 particles exhibit an extremely high adsorption toward RhB up to 98%, while at relatively high pH values above 7.1, the dye adsorption onto Bi4Ti3O12 particles decrease substantially and the adsorption percentage is only about 8–13%. The significant effect of pH on the dye adsorption can be explained by the pH-induced change in the surface charge of Bi4Ti3O12 particles. The isoelectric point of Bi4Ti3O12 is located at pH = 4.4;20) that is, Bi4Ti3O12 particles bear a positive surface charge when pH < 4.4 and a negative surface charge when pH > 4.4. This indicates that at low pH values the positively charged Bi4Ti3O12 particles exert an electrostatic attraction toward RhB anions, consequently resulting in a large adsorption of the dye; whereas at relatively high pH values the negatively charged Bi4Ti3O12 particles exert an electrostatic repulsion toward RhB anions, thus making the dye adsorption decrease significantly. The degradation percentage of RhB after 6 h of photocatalysis under different pH values is shown in Fig. 4(b). It is seen that the dye degradation reaches a very high level of 94.6%–99.7% at pH values below 7.1, but is decreased to 33.8%–44.8% when increasing pH over the range of 10.3–13.8. The high photocatalytic efficiency under low pH conditions might be attributed to the large adsorption effect and increased oxidation capacity due to attack of photogenerated holes.20)
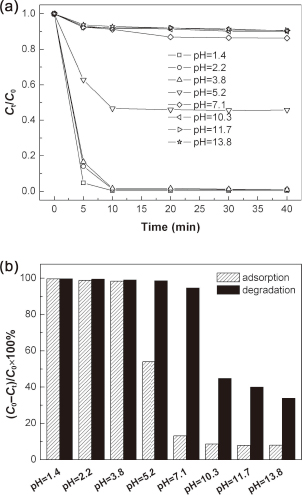
(a) Time-dependent adsorption of RhB onto Bi4Ti3O12 catalyst (sample S1) under different pH values in the dark. (b) Adsorption percentage of RhB after 20 min of adsorption and degradation percentage of RhB after 6 h of photocatalysis under different pH values.
For most of the photocatalysis systems, superoxide (•O2−), •OH and h+ are thought to the dominant active species responsible for the pollutant degradation.23) It is known that ethanol, benzoquinone (BQ) and ammonium oxalate (AO) can be used as the scavengers of •OH, •O2− and h+, respectively.24) By investigating their effect on the photocatalytic degradation of RhB over Bi4Ti3O12, we can clarify the role of those active species in the photocatalysis. Figure 5 shows the photocatalytic degradation of RhB after 6 h irradiation over the Bi4Ti3O12 catalyst (sample S1) by adding 10 mL of ethanol, 0.1 mmol of BQ and 1 mmol of AO in 100 mL reaction solution, as well as without adding the scavengers. In every case, the reaction solution is fixed at the normal pH value of 7.1. It is found that when adding ethanol and BQ to the reaction solution, the photocatalytic degradation of RhB still maintains a high level, reaching 92.8% and 93.2%, repectively. This implies that the addition of ethanol and BQ has a negligible suppression on the dye degradation, and •OH and •O2− are not the main active species in the photocatalysis. However, the addition of AO leads to a substantial decrease in the dye degradation and only 27.2% of RhB is observed to be degraded. As the addition of AO simultaneously results in the quenching of h+,24) we therefore suggest that h+ plays the dominant role in the dye degradation.
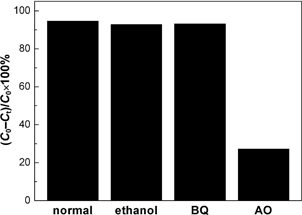
Effect of ethanol, BQ and AO on the RhB degradation over the Bi4Ti3O12 catalyst (sample S1) after 6 h irradiation.
We also examined the •OH radicals by spectrofluorimetry using TPA as the •OH scavenger. Figure 6 show the PL spectra of the TPA solution reacted for different times over the Bi4Ti3O12 catalyst (sample S1) at the normal pH value of 7.1. It is seen that the PL spectra of the reacted TPA solution are very similar to the fresh TPA solution, where no obvious PL signal is observed at around 429 nm. This indicates that almost no •OH radicals are produced over the simulated-sunlight-irradiated Bi4Ti3O12 catalyst.
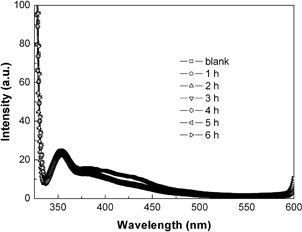
PL spectra of the TPA solution reacted for different times over the Bi4Ti3O12 catalyst (sample S1).
We also compare the photocatalytic activity of the prepared Bi4Ti3O12 samples S1–S4 toward the degradation of RhB at the normal pH value of 7.1. Figure 7(a) shows the plots of ln(Ct/C0) versus irradiation time (t) for the samples. It is seen that the photocatalytic degradation of RhB can be well modeled using the first-order kinetic equation ln(Ct/C0) = −kappt, where kapp is the apparent first-order reaction rate constant (min−1).25) The rate constant kapp for the samples follows the sequence: S1 > S2 > S3 > S4. Figure 7(b) shows the degradation percentage of RhB after irradiation for 6 h and the adsorption percentage of RhB after 20 min of adsorption in the dark. For samples S1–S4, the degradation percentage of the dye is about 94.6%, 93.5%, 83.1% and 75.6%, respectively. It is obvious that the photocatalytic activity of Bi4Ti3O12 increases with the decrease in particle size. This can be attributed to the fact that for smaller-sized photocatalyst, the photogenerated electrons and holes migrate more easily to the photocatalyst surface and furthermore more surface active sites are available for the photocatalytic reaction. The adsorption percentage of the dye for samples S1–S4 is about 13.1%, 12.6%, 9.8% and 7.4%, respectively, which exhibits a slight decrease trend with the increase in particle size.
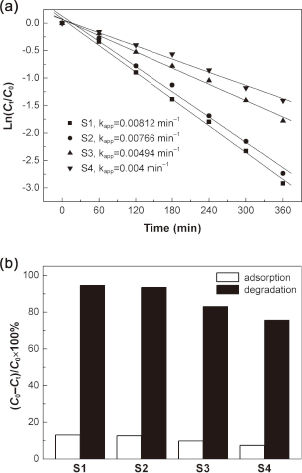
(a) Plots of ln(Ct/C0) versus irradiation time (t) for Bi4Ti3O12 samples. (b) Degradation percentage of RhB after irradiation for 6 h and adsorption percentage of RhB after 20 min of adsorption in the dark.
Based on a polyacrylamide gel route, single-phase Bi4Ti3O12 nanoparticles with sphere-like or ellipsoid-like shapes were prepared separately using citric acid, acetic acid, tartaric acid and EDTA as the chelating agent. The samples prepared using the four chelating agents have an average size of 32, 35, 90 and 120 nm, respectively. The bandgap energy of the samples is obtained to be 3.27 eV. The pH value of the solution has an important influence on the adsorption and photocatalytic degradation of RhB upon Bi4Ti3O12 nanoparticles. A large adsorption and degradation of the dye is observed at relatively low pH values, especially under acidic conditions. The addition of ethanol and BQ has a negligible suppression on the dye degradation, but the addition of AO leads to a substantial decrease in the dye degradation. Furthermore, no •OH radicals are observed to be produced over the simulated-sunlight-irradiated Bi4Ti3O12 catalyst. This suggests that h+ plays the dominant role in the dye degradation.
This work was supported by the National Natural Science Foundation of China (Grant No. 51262018) and the Hongliu Outstanding Talents Foundation of Lanzhou University of Technology (Grant No. J201205).