2016 Volume 57 Issue 10 Pages 1853-1856
2016 Volume 57 Issue 10 Pages 1853-1856
Mg-Li system alloys have poor corrosion properties, especially cold-rolled sheet leads to exfoliation corrosion as a result of long-term immersion test. Addition of aluminum element to Mg-14 mass% Li alloy could be suppressed the occurrence of exfoliation corrosion. However, excess addition of aluminum to Mg-14 mass% Li alloy causes the degradation of corrosion resistance. In this study, the composition of aluminum in Mg-14 mass% Li was optimized to improve the corrosion property. 3 mass% of Al addition could be suppressed the corrosion rate because enough aluminum element dissolved into matrix and precipitation of the second phase was inhibited.
Light weight metal with high specific strength are desired for transportation industry to apply as a housing material. Mg alloy has been improved the mechanical property and corrosion resistance. Hcp-structured Mg alloy has high strength by additional rare-earth element such as Y, Gd, Nd and so on1–3). However, these alloys could not be applied the cold working at room temperature such as rolling, extrusion and deep drawing. Mg-Li system alloys also has excellent cold-workability compared to commercial hcp-structured Mg alloys4). However, Mg-Li alloys have poor corrosion resistance because not only that is Mg-based alloy but Li as a major alloying element has the lowest standard electrode potential in the metal elements. The number of studies about corrosion protection for Mg-Li alloys has been reported5–7). These corrosion protective coatings are effective to prevent the metal surface from exposing to the corrosion environment. Unfortunately, while the coating layer is broken by some kind of factor, the corrosion reaction proceeds rapidly into the metal matrix because Mg-Li alloy is sensitive to corrosion. The exfoliation corrosion especially develops in the cold-rolled Mg-Li alloy with proceeding the corrosion reaction. Therefore, it is important to improve the corrosion resistance of Mg-Li alloy for the practical application in the industrial products. The authors have been studied about the corrosion behavior of Mg-14 mass%Li-1 mass% Al (LA141) alloy and demonstrate the corrosion rate of this alloy could be decreased by controlling the microstructure and impurity contamination8,9). In the previous study, it suggested that the exfoliation corrosion was caused by the development of the localized corrosion around the grain boundary. This indicates the corrosion progression in Mg-Li alloy could be suppressed by microstructural design such as dispersion of the second phase particle and the constitution of the matrix. It is important to investigate the initial stage of corrosion for improvement the corrosion resistance of Mg-Li system alloy.
The additional alloying elements for Mg-Li system alloy have been reported to improve mechanical properties and the corrosion resistance10–12). Particularly, aluminum is major alloying element for Mg-Li alloy to secure enough strength and corrosion performances. For example, LA141 (Mg-14 mass% Li-1 mass% Al) was established in 1960's13) and it is recently reported the yield strength was achieved to >220 MPa by combination of 5 mass% addition of aluminum and grain refinement14). Mg-Li alloy with high Al content could be controlled the microstructural characteristic by heat treatment. According to phase diagram of Al-Li-Mg system, Mg-14 mass% Li with several percent of Al alloy plays the coexistence state of Li solid solution matrix with AlLi phase at 200℃, although that is single phase of Li solid solution at 400℃15). It is important the behavior of second phase in Mg alloy because precipitates cause the local galvanic corrosion or preferential anodic dissolution of either phase16). Therefore, microstructural features are able to be changed by heat treatment such as solution treatment and aging, and these microstructures may influence on corrosion property of Mg-Li system alloys. In this study, the effect of microstructural characteristics on the corrosion behavior of Mg-14 mass% Li-x mass% Al (x = 1, 2, 3 and 5) alloy was investigated to improve the corrosion resistance of Mg-Li-Al alloy.
Mg-14 mass% Li with several percent of Al alloys were prepared by remelting of mixture of Mg-14 mass% Li-1 mass% Al and Mg-14 mass% Li-9 mass% Al alloys. The target compositions were Mg-14 mass% Li-x mass% Al (x = 1, 2, 3 and 5, respectively). The master alloys were put into MgO crucible with the size of ø30 × 60 mm and melted at 750℃ in Ar atmosphere using a mullite tube furnace. Melt was kept for 3 hours at 750℃, with bubbling of Ar gas using stainless tube. After cooling in the furnace to room temperature, all specimens were homogenized at 300℃ for 1 hour in the furnace. All homogenized specimens were analyzed by GD-OES for the chemical composition of Li and Al in the alloy. Chemical compositions of these alloys were listed in Table 1.
| Specimen | Li | Al | Mg |
|---|---|---|---|
| Mg-14 Li-1 Al | 13.7 | 0.87 | Bal. |
| Mg-14 Li-2 Al | 13.2 | 2.21 | Bal. |
| Mg-14 Li-3 Al | 13.8 | 3.37 | Bal. |
| Mg-14 Li-5 Al | 13.7 | 5.31 | Bal. |
| (in mass%) |
The specimens for evaluations of the microstructure and corrosion behavior were prepared by cutting to 10 × 10 × 2 mm and mounting into epoxy resin with copper lead. The copper lead was connected by conductive silver paste (Fujikura Kasei Co. Ltd., Dotite® D-550) to the specimen and shielded by PTFE tube to avoid short-circuit through the electrolyte. Microstructures were observed before and after corrosion immersion test using Nikon MA100 optical microscope and JEOL JCM-6000 scanning electron microscope. Only the specimens before corrosion test were etched by 5 vol% nitric acid in methanol. Corrosion test for microstructural observation was performed in 5 mass% NaCl aqueous solution with saturated Mg(OH)2 for 30 min at room temperature to investigate the initial stage of corrosion. The electrochemical polarization measurement was performed using Hokuto Denko HSV-110 automatic polarization system with Ag/AgCl reference electrode and 100 × 50 × 0.5 mm stainless steel plate as a counter electrode. The potential sweep rate was 100 mV min−1 and swept to ±1.0 V from OCP after 5 min of immersion to NaCl solution.
To investigate the local galvanic corrosion between matrix grain and the second phase particles, AlLi intermetallic alloy was prepared by melting of Al and Li metal grains in Ar atmosphere at 750℃. Corrosion potential of AlLi alloy was also measured from the polarization curve in 5 mass% NaCl aqueous solution. Also, Mg-14 mass% Li-3 mass% Al alloy was solution heat treated at 370℃ for 5 and 10 hours to change the distribution of second phase. The volume fractions of AlLi intermetallic phase in Mg-14 mass% Li-3 mass% Al alloy were measured by using an image analysis software for specimens with some different conditions of solution heat treatment to alter distribution of the second phase.
All specimens consisted mostly of bcc-structured Li solid solution phase. XRD patterns of Mg-14 mass% Li-x mass% Al (x = 1, 2, 3 and 5) alloys were shown in Fig. 1. Alloys with more than 2 mass% of Al, the second phase of AlLi was detected. In addition, Mg-14 mass% Li-5 mass% Al alloy has small amount of Mg17Al12 phase. As a result of microstructural observation, the alloy with 1 mass% Al also has small amount of AlLi particle. Figures 2(a)–(d) show the microstructures of homogenized specimens. Fine spherical particles of the second phase were observed in the grain boundary of matrix of Mg-14 mass% Li-1 mass% Al alloy. As increasing the amount of Al concentration in the specimen, the volume fraction of the second phase increased and they had network-like distributions.
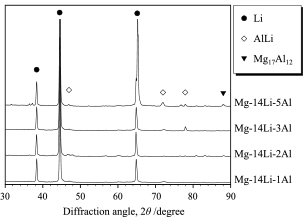
XRD patterns of homogenized Mg-14 mass% Li-x mass% Al alloys.
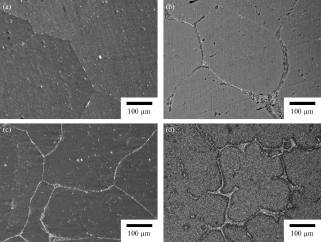
Initial microstructures of homogenized Mg-14 mass% Li- x mass% Al alloys. (a) Mg-14Li-1Al and (b) Mg-14Li-2Al, (c) Mg-14Li-3Al and (d) Mg-14Li-5Al.
Figure 3(a) shows the polarization curves of homogenized Mg-14 mass% Li alloys. The corrosion potential (Ecorr) and the calculated corrosion rate (v) were measured from the polarization test. The corrosion rate was calculated as with previous report9) by following equation;
| \[\begin{split} \boldsymbol{\nu} = &(i_{\mathrm{corr}} /96500) \\& \times (M_{\mathrm{Mg}} \times (1/2) \times c_{\mathrm{Mg}} + M_{\mathrm{Li}} \times c_{\mathrm{Li}} + M_{\mathrm{Al}} \times (1/3) \times c_{\mathrm{Al}}) \end{split} \] |
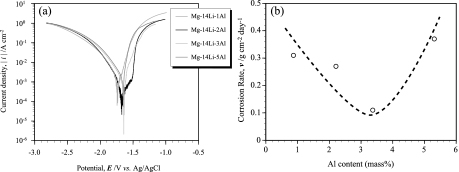
(a) Polarization curves of homogenized Mg-14 mass% Li alloys and (b) the relationship between corrosion rate and Al content in Mg-14 mass% Li alloy.
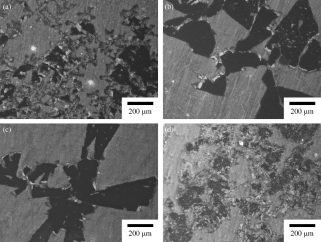
Microstructures of Mg-14 mass% Li-x mass% Al alloys after immersion test for 30 min. (a) Mg-14Li-1Al and (b) Mg-14Li-2Al, (c) Mg-14Li-3Al and (d) Mg-14Li-5Al.
As a result of XRD analysis, the prepared intermetallic alloy consisted of only AlLi phase. Corrosion potentials of Mg-14 mass% Li with several Al containing alloy and AlLi intermetallic alloy were listed in Table 2. The corrosion potential of Mg-14 mass% Li alloys was increased with increasing Al content. Contrary, AlLi intermetallic alloy has less noble potential than Mg-14 mass% Li alloys. The corrosion potential of solid solution alloy generally increases with increasing the content of additional solute element with noble potential17). It is reasonable to explain the corrosion potential varies with Al content in Mg-14 mass% Li alloys. However, it cannot be explained in the alloys with second phase particles. Actually, Mg-14 mass% Li-5 mass% Al alloy has same value of corrosion potential with Mg-14 mass% Li-2 mass% Al alloy. This means that there was a local galvanic corrosion between matrix grain and the second phase of AlLi particles. The microstructure of solution treated Mg-14 mass% Li-3 mass% Al alloy after corrosion test was shown in Fig. 5(a). The intermetallic phase at the grain boundary of matrix was dissolved because of galvanic corrosion. On the other hand, the outer part of matrix grain has flat surface because there was not corrosion reaction but inner part of the grain represents many dimples due to the evolution of hydrogen gas. In Mg-Li alloys, it is subject to the anodic dissolution of the matrix grain spontaneously in aqueous solution because it has less noble potential, so there might be both anodic and cathodic reactions in the matrix grain. The outer part of matrix grains has smaller content of Al than the inner part due to neighboring AlLi phase. This might be cause the difference of electrode potential between the outer part of matrix grain and the inner part. These results suggest that the excessive amount of AlLi phase degrades the corrosion resistance of Mg-Li-Al alloy despite of high Al content. Figure 5(b) shows the relationship between the volume fraction of AlLi phase and corrosion rate in the Mg-14 mass% Li-3 mass% Al alloy with solution treatment. Unfortunately, solution treatment for 10 h could not be obtained fully solid solution alloy. The volume fractions of AlLi phase were 5.2, 3.9 and 3.4% for the specimens of as-homogenized, 5 h and 10 h at 300℃ of solution treated alloy, respectively. The corrosion rate increases with increasing the volume fraction of AlLi phase. Also, it is acceptable to similar tendency about the corrosion potential as listed in Table 2. The corrosion resistance is increased by the solution of Al into the matrix and the shrinking the AlLi phase. These results indicate the corrosion property of Mg-14 mass% Li alloy could be improved by addition of appropriate amount Al without precipitates as a second phase. Therefore, the solution treatment for the alloy with excess Al is effective method to improve the corrosion property when it could be completely dissolved into the matrix.
| Specimen | Condition | Corrosion Potential, Ecorr/V vs. Ag/AgCl | Calculated Corrosion Rate, v/g cm−2 day−1 |
|---|---|---|---|
| Mg-14 Li-1 Al | Homogenized | −1.71 | 0.31 |
| Mg-14 Li-2 Al | Homogenized | −1.67 | 0.27 |
| Mg-14 Li-3 Al | Homogenized | −1.68 | 0.11 |
| ST for 5 h | −1.66 | 0.07 | |
| ST for 10 h | −1.62 | 0.04 | |
| Mg-14 Li-5 Al | Homogenized | −1.67 | 0.37 |
| AlLi | As-cast | −2.03 | not measured |
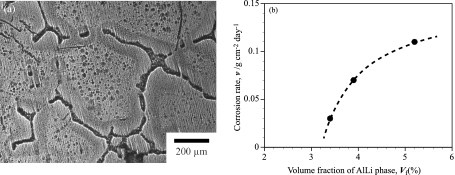
(a) Microstructure of Mg-14 mass% Li-3 mass% Al alloy with solution treatment (for 5 h) after corrosion test and (b) the relationship between the volume fraction of AlLi phase and corrosion rate in the Mg-14 mass% Li-3 mass% Al alloy with solution treatment.
The corrosion property of Mg-14 mass% Li with several content of Al was investigated in this study. Mg-14 mass% Li-3 mass% Al alloy has the highest corrosion resistance due to the solute Al into matrix. The main precipitates of AlLi phase has less noble electrode potential about −2.0 V vs. Ag/AgCl. Excess precipitation of AlLi causes the degradation of corrosion rate because preferential anodic dissolution of AlLi results a local galvanic corrosion with matrix grain as a cathode. Appropriate amount of Al addition could improve the corrosion property because the matrix with enough Al concentration as a solute element increased the electrode potential. These results provided that it is also possible to improve the corrosion rate of these alloys by solution treatment.
This work was partly supported by 34th research grant from Suga Weathering Technology Foundation (2015).