2016 Volume 57 Issue 12 Pages 2153-2157
2016 Volume 57 Issue 12 Pages 2153-2157
A Bi2Te3–Sb2Te3 solid solution was prepared by mechanical alloying (MA) followed by hot pressing (HP). X-ray diffraction indicated that all samples which were removed at a depth below the surface of approximately 1 mm were single-phase and isotropic Bi2Te3–Sb2Te3 solid solution. Reduction of the phonon thermal conductivity as a result of the fine-grains caused by MA predominated over the solid-solution effect caused by melt growth. The Seebeck coefficient and electrical and thermal conductivities fluctuated between those for (Bi2Te3)0.15(Sb2Te3)0.85 and (Bi2Te3)0.2(Sb2Te3)0.8 at room temperature. A (Bi2Te3)0.15(Sb2Te3)0.85 solid solution with a dimensionless figure of merit ZT = 1.16 at 367 K was obtained by MA–HP. These results indicate that the maximum ZT of the Bi2Te3–Sb2Te3 solid solution obtained by MA–HP was not restricted to a composition of (Bi2Te3)0.25(Sb2Te3)0.75, which has the minimum phonon thermal conductivity in the case of melt growth.
Bi2Te3–Sb2Te3 solid solutions are the best p-type thermoelectric semiconductors near room temperature, and are widely used for refrigerators and research generators1,2). They are eco-materials and recover exhaust waste heat well at near room temperature2). The performance of a thermoelectric semiconductor is defined by the dimensionless figure of merit ZT:
| \[ZT = \alpha^2 \sigma \kappa^{ - 1} T\] | (1) |
| \[ \kappa_{\rm total} = \kappa_{\rm phonon} + \kappa_{\rm carrier} = \kappa_{\rm phonon} + L \sigma T \] | (2) |
A Bi2Te3–Sb2Te3 solid solution has a rhombohedral crystal structure with the R3m space group, and anisotropic physical and thermoelectric properties1). Bi2Te3–Sb2Te3 solid-solution materials are anisotropic. The anisotropies of the electrical and phonon thermal conductivities of Bi2Te3 are respectively estimated to be σ∥/σ⊥ ≈ 3 and κ∥/κ⊥ ≈ 0.47, for the values parallel and perpendicular to the c-basal plane, but the Seebeck coefficient is isotropic5).
The total thermal conductivity is decreased by reductions in the phonon thermal conductivity caused by the solid-solution effect arising from mass difference scattering1). For example, the phonon thermal conductivity of a silicon-germanium solid-solution semiconductor produced by melt growth decreases with increasing mixing ratio6). The minimum phonon thermal conductivity of a Bi2Te3–Sb2Te3 solid solution formed by melt growth occurs at a composition of around (Bi2Te3)0.25(Sb2Te3)0.757). The reduction in phonon thermal conductivity caused by the solid-solution effect determines the Bi2Te3–Sb2Te3 composition that gives the best thermoelectric figure of merit6,7). The best thermoelectric figure of merit for a Bi2Te3–Sb2Te3 solid solution formed by melt growth was achieved at a composition of (Bi2Te3)0.25(Sb2Te3)0.752,3).
Mechanical alloying (MA) is also used for the preparation of thermoelectric materials1,4,8,9). MA has a refining effect because of boundary scattering10). To maintain a low phonon thermal conductivity, fine-grained materials are normally prepared using a powder metallurgy method such as MA4). MA followed by hot pressing (HP) can be used to synthesize materials that remain as solid solutions. Sintered compact MA powders have random crystal orientations and refined structures, which decrease the phonon thermal conductivity.
In MA–HP processes, reduction of the phonon thermal conductivity is simultaneously caused by solid-solution and fine-grain effects10–12). It is not clear whether the main cause of the reduced phonon thermal conductivity is the solid-solution or fine-grain effect13).
The preferred orientation also affects the electrical and phonon thermal conductivities on the surface of a Bi2Te3–Sb2Te3 solid solution, as a result of thermal stress at the heterojunction between the HP mold and sintered compact in Bi2Te3–Sb2Te3 solid-solution formation using a hot deformation process14–17).
In the present study, a Bi2Te3–Sb2Te3 solid solution was prepared by MA–HP without the influence of a preferred orientation, because a preferred orientation causes anisotropies of the electrical and thermal conductivities. The reduced phonon thermal conductivity caused by the solid-solution effect was investigated based on the effect of the Bi2Te3–Sb2Te3 composition ratio.
Bismuth (99.999%), antimony (99.999%), and tellurium (99.9999%) were weighed to give stoichiometries of (Bi2Te3)x(Sb2Te3)1−x (x = 0.05–0.5). Only millimeter-scale grains of the raw materials were used, to suppress contamination by a surface oxide layer. The raw materials were placed in a stainless-steel vessel with a milling ball made of a silicon nitride ceramic in a glove box filled with argon. MA was performed in a Fritch P-5 planetary ball mill at a maximum speed of 180 rpm for 30 h. The mechanically alloyed powder was passed through a 150-μm diameter sieve and it was confirmed that no raw materials remained. The mechanically alloyed powder was sintered by HP at 623 K under a uniaxial pressure of 147 MPa in an argon atmosphere. The sintered compacts were cylinders of diameter 10 mm and height 9 mm. These compacts were cut into disks approximately 1 mm and 10 mm in diameter to obtain isotropic disks. Approximately 1 mm of material was removed from the top and bottom of the sintered compact surface, because it has been reported that this promotes formation of the (00∙l) texture14).
The out-of-plane direction of the disks was examined by X-ray diffraction (XRD; Rigaku Multiflex) using Cu Kα radiation in the Bragg angle range 2θ = 20–80° with a step size of 0.1° degree and a step speed of 1.0 s per step. The orientation factor F for evaluating the isotropy was determined using the Lotgering method:
| \[F \equiv \frac{(D - D_0)}{1 - D_0}\] | (3) |
| \[D = \frac{\sum I_{00l} }{\sum I_{hkl}}\] | (4) |
The Seebeck coefficients, electrical conductivities, and thermal conductivities at room temperature of all the disks were measured, and their power factors α2σ and dimensionless figures of merit ZT were estimated. The Seebeck coefficients were measured using the constructed thermal contact method20), and the thermal conductivity was determined using the constructed static comparison method2,21). The electrical conductivity was measured by the four-point probe method using an electrical resistance system based on a 2182A/6220 instrument (Keithley Instrument, Inc.). The probe size was 1.0 mm and it was made of tungsten carbide. All measurements made using the electrical conductivity system were confirmed by ohmic contact21). The accuracies of the Seebeck coefficient was less than ±2% and electrical conductivity and thermal conductivity were less than ±1%, respectively20,22). The figures of merit of the (Bi2Te3)x(Sb2Te3)1−x materials were determined at room temperature, based on the temperature dependences of the Seebeck coefficient and electrical conductivity from 300 to 573 K, determined using a ZEM-3 instrument (Advance-Riko). (Bi2Te3)x(Sb2Te3)1−x specimens of dimensions 10 mm × 10 mm × 9 mm were sintered using MA–HP. A piece with the dimensions 3.0 mm × 3.0 mm × 8.0 mm was removed from the center of the sintered specimen to eliminate the influence of anisotropy. The ZEM-3 instrument had an accuracy of less than ±7%; the dimensionless figure of merit ZT was estimated from the temperature dependences of the Seebeck coefficients and electrical conductivities obtained using the ZEM-3 instrument, and the thermal conductivity at room temperature.
The obtained disks were p-type conductors and optical microscopy showed that they were dense materials.
Figure 1 shows the XRD patterns of disks of (Bi2Te3)x(Sb2Te3)1−x (x = 0.05, 0.125, 0.15, and 0.5) prepared using MA–HP. The main hkl and 00l reflections showed that the material was a Bi2Te3–Sb2Te3 solid solution. All the diffraction peaks indicated a single-phase of Bi2Te3-Sb2Te3 solid solution. No peaks were observed other than those from bismuth, antimony, and tellurium, and other compounds. The D and F values for (Bi2Te3)0.5(Sb2Te3)0.5 were estimated, using eqs. (3) and (4), to be 0.0192 and 0.0081, respectively, in the 2θ range 20–80°. A preferred orientation in the (00∙l) direction was not observed for the obtained disks. These results show that all the disks were single-phase, isotropically oriented Bi2Te3–Sb2Te3 solid solutions; isotropic disks had been obtained from MA–HP sintered compacts from which 1 mm of material had been eliminated from the top and bottom surfaces.

XRD patterns of (Bi2Te3)x(Sb2Te3)1−x (x = 0.05, 0.125, 0.15, and 0.5) disks produced using MA–HP.
Figure 2 shows the relationship between the electrical conductivity σ and x for (Bi2Te3)x(Sb2Te3)1−x at room temperature. The electrical conductivity σ gradually decreased with increasing Bi2Te3 content. This trend is similar to that of the electrical conductivity of a material synthesized using the melt-growth method23,24). However, fluctuations in the electrical conductivity were observed at around x = 0.2; such fluctuations have not been reported in the literature.

Relationship between electrical conductivity σ and x for (Bi2Te3)x(Sb2Te3)1−x at room temperature.
Figure 3 shows the relationships among the total, phonon, and carrier thermal conductivities and x for (Bi2Te3)x(Sb2Te3)1−x (x = 0.05–0.5). The phonon and carrier thermal conductivities were estimated using eq. (2) with the Lorenz number 2.45 × 10−8 W S−1 K−2. The total thermal conductivity decreased up to x = 0.3, and was constant for x > 0.3.

Relationships among total, phonon, and carrier thermal conductivities and x for (Bi2Te3)x(Sb2Te3)1−x.
Fluctuations in the total thermal conductivity κtotal were observed at around x = 0.2, consistent with the behavior of the electrical conductivity (Fig. 2). The total thermal conductivity seemed to be decreased because of a reduction in the phonon thermal conductivity caused by the solid-solution effect. The carrier thermal conductivity κcarrier decreased with increasing Bi2Te3 content. The trend in the carrier thermal conductivity corresponded to that in the electrical conductivity (Fig. 2), based on eq. (2). The phonon thermal conductivity κphonon increased with increasing Bi2Te3 content and did not correspond to the reduction in the thermal conductivity caused by the solid-solution effect in melt growth25). In the case of the melt-growth method, the phonon thermal conductivity decreased with increasing Bi2Te3 content because of the solid-solution effect. There is clearly a minimum composition close to (Bi2Te3)0.25(Sb2Te3)0.757). In the case of the MA–HP method, a composition of (Bi2Te3)0.25(Sb2Te3)0.75 was not the minimum for phonon thermal conductivity.
The reduction in the phonon thermal conductivity caused by the fine-grain effect caused by MA was more important than that caused by the solid-solution effect in melt growth. Thermoelectric materials with compositions around (Bi2Te3)0.25(Sb2Te3)0.75 have been developed for many applications because they have the advantage of low phonon thermal conductivity because of melt growth2). The predicted maximum thermoelectric figure of merit was not close to that of (Bi2Te3)0.25(Sb2Te3)0.75 produced by MA–HP.
The relationship between the Seebeck coefficient α and x for (Bi2Te3)x(Sb2Te3)1−x (x = 0.05–0.5) is shown in Fig. 4. All the disks showed p-type conduction at room temperature. The Seebeck coefficient increased as x increased from 0.05 to 0.15. Fluctuations appeared between x = 0.15 and x = 0.2, but the coefficient was constant, at 220 × 10−6 V K−1, at x = 0.25 to 0.5. These fluctuations were the opposite of those seen in Fig. 2. The fluctuations between x = 0.15 and x = 0.2 are consistent with the trend in the electrical conductivity shown in Fig. 2, because of the inverse relationship between the Seebeck coefficient α and the electrical conductivity σ. Such fluctuations have not been reported in the literature. The band structure of (Bi2Te3)x(Sb2Te3)1−x may change, as previously reported25).

Relationship between Seebeck coefficient α and x for (Bi2Te3)x(Sb2Te3)1−x (x = 0.05–0.5).
Figure 5 shows the relationship between the power factor α2σ and x for (Bi2Te3)x(Sb2Te3)1−x (x = 0.05–0.5). The power factor increased as x increased from 0.05 to 0.125, and decreased above x = 0.125, i.e., with increasing Bi2Te3 content. The power factor at x = 0.125 was 3.5 × 10−3 W m−1 K−2. The effects of fluctuations in the electrical conductivity (Fig. 2) and Seebeck coefficient (Fig. 4) were small and the power factor at x = 0.2 was 2.38 × 10−3 W m−1 K−2.

Relationship between power factor α2σ and x for (Bi2Te3)x(Sb2Te3)1−x (x = 0.05–0.5).
Figure 6 shows the relationship between the dimensionless figure of merit ZT and x for (Bi2Te3)x(Sb2Te3)1−x (x = 0.05–0.5). ZT increased up to x = 0.15. The fluctuations between x = 0.15 and x = 0.2 were consistent with the behaviors of the Seebeck coefficient, and electrical and thermal conductivities. The maximum ZT, at x = 0.15, was 0.99 at room temperature.

Dimensionless figure of merit ZT versus x plot for (Bi2Te3)x(Sb2Te3)1−x: (x = 0.05–0.5).
The temperature dependences of the Seebeck coefficients and electrical conductivities of rectangular samples of (Bi2Te3)0.15(Sb2Te3)0.85 and (Bi2Te3)0.125(Sb2Te3)0.875, which had higher power factor at room temperature, were determined.
Figure 7 shows the Seebeck coefficient versus temperature plots for (Bi2Te3)0.15(Sb2Te3)0.85 and (Bi2Te3)0.125(Sb2Te3)0.875. The Seebeck coefficients were constant up to 450 K and decreased above 450 K. The Seebeck coefficient of (Bi2Te3)0.15(Sb2Te3)0.85 was slightly higher than that of (Bi2Te3)0.125(Sb2Te3)0.875 up to 475 K. Above 475 K, the Seebeck coefficient of (Bi2Te3)0.15(Sb2Te3)0.85 was slightly lower than that of (Bi2Te3)0.125(Sb2Te3)0.875. This could be because of band gap differences, because the band gaps of Bi2Te3 and Sb2Te3 are 0.13 and 0.28 eV, respectively26). The band gap of (Bi2Te3)0.125(Sb2Te3)0.875 is wider than that of (Bi2Te3)0.15(Sb2Te3)0.85. The maximum Seebeck coefficient of a wide band gap semiconductor shifts to a higher temperature24).

Seebeck coefficient versus temperature plots for (Bi2Te3)0.15(Sb2Te3)0.85 and (Bi2Te3)0.125(Sb2Te3)0.875.
Figure 8 shows plots of the electrical conductivities of (Bi2Te3)0.15(Sb2Te3)0.85 and (Bi2Te3)0.125(Sb2Te3)0.875 versus temperature. The electrical conductivity decreased with increasing temperature. These (Bi2Te3)x(Sb2Te3)1−x (x = 0.05–0.5) solid-solution materials synthesized using MA–HP had the conductive properties of a degenerate semiconductor.

Plots of electrical conductivities of (Bi2Te3)0.15(Sb2Te3)0.85 and (Bi2Te3)0.125(Sb2Te3)0.875 versus temperature.
Figure 9 shows plots of the power factors of (Bi2Te3)0.15(Sb2Te3)0.85 and (Bi2Te3)0.125(Sb2Te3)0.875 versus temperature. The power factor of (Bi2Te3)0.15(Sb2Te3)0.85 was slightly higher than that of (Bi2Te3)0.125(Sb2Te3)0.875 up to 475 K. Above 475 K, the power factor of (Bi2Te3)0.15(Sb2Te3)0.85 was lower than that of (Bi2Te3)0.125(Sb2Te3)0.875. These behaviors are consistent with the temperature dependence of the Seebeck coefficient. The power factors decreased with increasing temperature. The maximum power factor for (Bi2Te3)0.15(Sb2Te3)0.85 was 3.41 × 10−3 W m−1 K−2 at 343 K and for (Bi2Te3)0.125(Sb2Te3)0.875 was 3.08 × 10−3 W m−1 K−2 at 300 K.
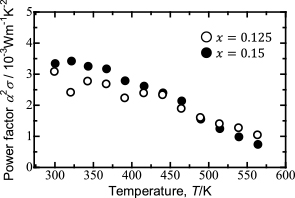
Plots of power factors of (Bi2Te3)0.15(Sb2Te3)0.85 and (Bi2Te3)0.125(Sb2Te3)0.875 versus temperature.
Figure 10 shows plots of the dimensionless figures of merit ZT for (Bi2Te3)0.15(Sb2Te3)0.85 and (Bi2Te3)0.125(Sb2Te3)0.875 versus temperature at a fixed total thermal conductivity κtotal at room temperature. It was presumed that because of the Wiedemann–Franz law and high-temperature Umklapp process the thermal conductivities of these materials would be approximately constant in this temperature range7). The dimensionless figure of merit ZT of (Bi2Te3)0.15(Sb2Te3)0.85 was higher than that of (Bi2Te3)0.125(Sb2Te3)0.875. For (Bi2Te3)0.15(Sb2Te3)0.85, ZT was 1.16 at 367 K. This value was slightly higher than the previously reported value, 1.1212).

Plots of dimensionless figures of merit ZT of (Bi2Te3)0.15(Sb2Te3)0.85 and (Bi2Te3)0.125(Sb2Te3)0.875 versus temperature with fixed total thermal conductivity κtotal at room temperature.
In the present study, isotropic Bi2Te3–Sb2Te3 solid solutions were prepared using MA–HP. These results are as follows.
These results indicate that the maximum ZT for Bi2Te3–Sb2Te3 solid solutions prepared using MA–HP was not restricted to a composition of (Bi2Te3)0.25(Sb2Te3)0.75, which is the composition for minimum phonon thermal conductivity of a material produced by melt growth.