2017 Volume 58 Issue 6 Pages 886-891
2017 Volume 58 Issue 6 Pages 886-891
A series of slow strain rate tests (SSRTs) were carried out on a Ti-6Al-4V alloy in hydrogen gas at high temperatures (up to 300℃) and high pressures (up to 75 MPa). In order to evaluate the degree of embrittlement of the titanium alloy in a hydrogen gas environment, measurements of tensile strength, elongation and reduction of area were normalized using results obtained in air of atmospheric pressure and in the same temperature range. Results show that Ti-6Al-4V alloy does not exhibit any decrease in tensile strength, elongation or reduction of area in a high pressure hydrogen gas environment.
Titanium, with excellent corrosion resistance and high specific strength, is used in a wide range of fields, including aircraft parts and in new applications such as parts for fuel cell vehicles. In fuel cell cars sold in 2015 titanium was used in fuel cell stacks1); if titanium can be used not only for fuel cell stacks that are exposed to low pressure hydrogen but also for pipes and valves exposed to hydrogen gas of high temperatures and high pressures, fuel cell vehicles can be made lighter in weight. Depending on conditions, however, titanium becomes brittle when used in a hydrogen environment due to the effect of hydrogen atoms penetrating through its surface2). The maximum hydrogen temperature and pressure in currently marketed fuel cell vehicles are approximately 85℃ and 70 MPa, respectively3). Accordingly, in order to examine the potential use of titanium in pipes and valves of fuel cell vehicles, there is a need to first verify that titanium materials would not become brittle in a hydrogen environment with a temperature and pressure equivalent to those under actual use.
Base on the Japanese Industrial Standards (JIS), in a range of strengths, namely Type 1 to Type 4, in addition to pure titanium there are a range of titanium alloys to which metals such as Al, Sn and V are added4). Such typical titanium alloys include Ti-5Al-2.5Sn (equivalent to ASTM Gr6), Ti-6Al-4V (JIS H 4600 Class 60, α+β alloy) and Ti-22V-4Al (JIS H 4600 Class 80, β alloy). The Ti-6Al-4V, in particular, excels in secondary workability and has favorable mechanical properties. With its excellent corrosion resistance and high strength, this alloy is widely used as structural and medical material in industries such as chemical, mechanical and transportation equipments. Consideration has been given to the critical hydrogen concentration5) at which Ti-6Al-4V starts to become brittle, and its embrittlement behavior in hydrogen gas. Testing on notched specimens in hydrogen gas of room temperature and approximately 45 MPa indicate that Ti-6Al-4V exhibits tensile strength of approximately 75% of that in an inert gas environment, but no great change in reduction of area6). In an evaluation of resistance to high pressure hydrogen using rupture discs, its critical pressure in hydrogen gas has been shown to be approximately 70% of that in inert gas7). Although a number of studies have been carried out on the embrittlement behavior of Ti-6Al-4V at room temperature, its embrittlement behavior in hydrogen gas of high temperatures and high pressures has not been sufficiently investigated.
High specific strength is a desired attribute for materials used in equipment for which weight saving is a key requirement, such as fuel cell vehicles. While it has no high specific strength, SUS316L steel is often used3) for making equipment which handles hydrogen gas of high temperatures and high pressures due to its low hydrogen embrittlement (HE) susceptibility8). The lowest tensile strength for Ti-6Al-4V in the JIS standard is 895 MPa4), which is greater than 480 MPa9) of SUS316L, and its specific gravity is 4.4 g/cm3 10), which is less than 7.9 g/cm3 of SUS316L. This suggests that Ti-6Al-4V is a material with high specific strength.
It is known that the HE susceptibility of carbon steel and low-alloy steel increases in material strength11). At the same time, the HE susceptibility of Ti-6Al-4V is generally considered to be brought about by the formation and breaking of hydrides12,13). Some reports, however, suggest a possible effect of dissolved hydrogen on titanium, as is the case for steel materials14). If such an effect is large, Ti-6Al-4V, a high-strength alloy, may exhibit HE susceptibility. In this study, we carried out a series of slow strain rate tests (SSRTs) on Ti-6Al-4V in a high temperature and high pressure hydrogen gas environment, and compared the mechanical properties, fracture status and fracture surface configuration. The temperature and pressure of hydrogen gas were set at 100℃ and 75 MPa respectively, equivalent to the maximum temperature and pressure levels in fuel cell vehicles. In addition, the same test was carried out at 300℃ and 75 MPa, due to few reports of evaluation of the mechanical properties of Ti-6Al-4V in a temperature range higher than that anticipated for fuel cell vehicles.
Commercially available Ti-6Al-4V was used for testing materials. Table 1 shows the chemical composition and tensile strength of materials. 3 to 4 mm thick testing materials were used in an “as-received” condition without heat-treating, to produce flat tensile specimen to the dimensions given in Fig. 1. Parallel parts of tensile specimens were dry-polished with emery polishing paper of up to #600, and washed with acetone.
| (mass%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | C | H | Fe | O | Al | V | Ti | σ (MPa) |
|
| Ti-6Al-4V | 0.01 | 0.02 | 0.006 | 0.08 | 0.18 | 6.17 | 3.89 | bal. | 1007 |
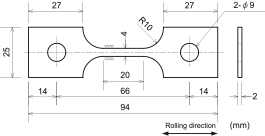
Shape and dimensions of the tensile specimen.
Figure 2 shows the schematic drawing of an SSRT device which enables testing in hydrogen gas of high temperatures and high pressures. Using this device, SSRTs were carried out in hydrogen gas at room temperature, 100℃ and 300℃ and at a pressure level of 75 MPa. The test procedures were as follows: after setting a tensile specimen in the pressure vessel, the vessel was pressurized to 3 MPa using N2 gas and then the pressure was released. This cycle was repeated three times. The same cycle was then repeated three times using H2 gas. Through these procedures, the air in the pressure vessel was replaced by N2 gas, which was then replaced by H2 gas. Following this, H2 gas was introduced into the pressure vessel, using a compressor, until the prescribed pressure was attained. In each case, pure H2 gas with a purity of 99.99999% or higher was used. It took approximately three hours from the onset of heating until the prescribed temperature and pressure were attained. SSRTs were started as soon as the prescribed temperature and pressure were attained. In each test, initial strain rate was set at 8.3 × 10−6 s−1. Elongation of the parallel part was regarded as that of the whole specimen, and the cross-head of the testing device was moved in the tensile direction at a speed of 0.17 µm/s. Immediately following the fracture of the specimen, heating was stopped, and the pressure vessel was subjected to natural cooling until its inside reached room temperature, which took approximately 12 hours. Once cooling was complete, hydrogen gas was released and the specimen was removed.

Schematic diagram of a slow strain rate testing (SSRT) apparatus at high temperature and high pressure environments.
From a series of SSRTs, tensile strength (σ), elongation (EL) and reduction of area (φ) were measured. EL was defined as the amount of crosshead displacement from application of tensile force till fracture. The effect of hydrogen gas on σ, EL and φ was evaluated using the relative values which were based on the measurements obtained in air with atmospheric pressure at the same temperature. As an example, σ was evaluated using the relative tensile strength σenv/σair, which is the ratio between σenv, tensile strength in a high temperature high pressure hydrogen gas environment, and σair, tensile strength in air with atmospheric pressure and at the same temperature. Similarly, EL and φ were evaluated using the relative elongation ELenv/ELair and the relative reduction of area φenv/φair, respectively. All of the σenv/σair, ELenv/ELair and φenv/φair values become smaller as the HE susceptibility increases. On the other hand, such values become 1 under no hydrogen effect (where no HE susceptibility expresses itself).
Fracture surfaces of specimens and sections of their parallel part after SSRTs were observed using a scanning electron microscope (SEM) and a optical microscope. The structure of sections was mirror-polished and etched with nitro-hydrofluoric acid (water 87%, nitric acid 10%, hydrofluoric acid 3%). At the same time, hydrogen contained in the parallel parts of fractured specimens was analyzed using thermal desorption spectrometry (TDS). The specimens were heated in vacuum from room temperature to 800℃ at a temperature increase rate of 10℃/min, and the amount of hydrogen desorbed from the specimens at each temperature level was analyzed with a quadrupole mass spectrometer (ST-200P Special Model made by ULVAC, Inc.). The apparent hydrogen content was then obtained by dividing the integral of hydrogen desorption curve by the mass of each sample subjected to analysis.
Figure 3 shows the macro configuration of fractured parts of the Ti-6Al-4V specimens resulting from SSRTs in 75 MPa hydrogen gas and in air, each at 100℃ and 300℃. All of the specimens, whether from the testing in air or hydrogen gas, demonstrated that fractures had occurred in a ductile manner exhibiting slight necking. No effects of air or hydrogen on the degree of necking were observed. In the above observation of macro configurations, no HE susceptibility was detected on any of the Ti-6Al-4V specimens subjected to 75 MPa hydrogen gas at 100℃ or 300℃. Similarly, observation of the macro configuration of the specimen subjected to an SSRT in room temperature 75 MPa hydrogen gas revealed that the specimen fractured in a ductile manner, suggesting no effects of the difference in environment, i.e. air or hydrogen.

The views of Ti-6Al-4V specimens fractured by SSRT in air and H2 gas at 100℃ and 300℃.
As examples of the stress-strain curve measured in SSRTs, Fig. 4 shows results obtained for Ti-6Al-4V tested in 75 MPa hydrogen gas and in air with atmospheric pressure, at 100℃ and 300℃. The tensile strength measured in 100℃ and 300℃ hydrogen gas was equivalent to that obtained in air at the same temperatures, with no effects of hydrogen being shown. At the same time, the elongation of Ti-6Al-4V at 100℃ was 19% in air and 17% in hydrogen gas, showing a decrease in the latter environment. The elongation was the same at 300℃ both in air and hydrogen gas.

Stress-strain curves of Ti-6Al-4V specimens. The SSRTs were carried out in air under the atmospheric pressure and H2 gas with 75 MPa, at 100°Cand 300℃ respectively.
Figure 5 shows the numerical values of σenv/σair, ELenv/ELair and φenv/φair at room temperature, 100℃ and 300℃. σenv, σair,ELenv and ELair were obtained from the stress-strain curve measured in SSRTs, and φenv and φair from the macro photographs of fracture surfaces taken from an angle in the tensile direction. The ratio σenv/σair of Ti-6Al-4V at room temperature, 100℃ and 300℃ ranged from 0.98 to 1.01, ELenv/ELair, from 0.87 to 0.98, and φenv/φair, from 0.96 to 1.06. These results indicate that, while a decrease was observed in elongation at 100℃, no effects of hydrogen were identified in tensile strength, in elongation or in reduction of area.

The relative intensities of the tensile strength (σ), the elongation (EL) and the reduction of area (φ) at RT, 100℃ and 300℃ with respect to those measured in air at the same temperatures but under atmospheric pressure.
Figure 6 shows the fracture surfaces of Ti-6Al-4V specimens subjected to SSRTs. Figure 6(a), Fig. 6(b) and Fig. 6(c) show the surfaces of fractures which occurred in 100℃ air with atmospheric pressure, in 100℃-75 MPa hydrogen gas, and in 300℃-75 MPa hydrogen gas, respectively. In all cases, whether air or hydrogen gas, fracture surfaces accompanying dimples indicate that fractures were of a ductile nature, with the same results for specimens in room temperature air and hydrogen gas environments. This indicates that Ti-6Al-4V was not embrittled by hydrogen in an environment of high pressure hydrogen gas at room temperature or high temperature.

SEM views of the fracture surfaces of Ti-6Al-4V specimens after SSRT. (a) In air under 100℃ and 0.1 MPa. (b) In H2 gas under 100℃ and 75 MPa. (c) In H2 gas under 300℃ and 75 MPa.
Sectional structures of post-SSRT Ti-6Al-4V specimens are shown in Fig. 7. Sectional structures at the center of the parallel part of each specimen (at a depth of approximately 1 mm from surface) were observed using a optical microscope. In both figures, the horizontal direction corresponds to the load direction for each specimen. No major differences were observed between the sectional structure in air with atmospheric pressure at 100℃ shown in Fig. 7(a) and that in 75 MPa hydrogen gas at 100℃ shown in Fig. 7(b). In addition, no hydrides such as those found in the case of pure titanium15) were observed. Sectional structures of specimens tested in 75 MPa hydrogen gas at room temperature and 300℃ did not exhibit any significant difference from those of specimens tested in air in the same temperature range. Thus the sectional structures of Ti-6Al-4V specimens did not suggest any effect of hydrogen in an environment of high pressure hydrogen gas at room temperature or high temperature.

Microstructures at the midsection of the parallel part of Ti-6Al-4V specimens after SSRT. (a) In air under 100°Cand 0.1 MPa. (b) In H2 gas under 100℃ and 75 MPa.
Figure 8 shows the results of TDS analyses of hydrogen contained in the parallel part of Ti-6Al-4V specimens after fracture. Analyses were performed on specimens subjected to SSRTs in 75 MPa hydrogen gas at 100℃ and 300℃, and in air with atmospheric pressure at 100℃. There was no desorption of hydrogen from any of the specimens at 350℃ or below. Hydrogen desorbed from every specimens, including those SSRTs in air, in the temperature range of 400 to 800℃. There were two peaks observed in each case of hydrogen desorption. In specimens tested in air, the first peak was at 600℃ and the second at near 750℃. For those tested in hydrogen gas, the first peak temperature was lower with the SSRT temperature increasing from 100℃ to 300℃, and being seen at around 550℃ in the latter case, where the volume of hydrogen desorption increased. The second peak was lower when the SSRT temperature increased to 300℃.

Hydrogen evolution curves of the parallel part of Ti-6Al-4V specimens after SSRT.
From the hydrogen desorption curve given in Fig. 8, it was confirmed that specimens subjected to SSRTs in air contained 50 ppm of hydrogen. We consider that this hydrogen was contained in those specimens prior to SSRTs, not having been absorbed from the environments during the tests. The hydrogen concentration in specimens tested in 75 MPa hydrogen gas at 100℃ was 86 ppm, having increased by 1.72 times from before the test. Specimens tested at a higher temperature, i.e. 300℃ demonstrated an even higher hydrogen concentration, 236 ppm, which was 4.72 times higher than before testing.
Although SSRTs were conducted in high pressure hydrogen gas with 75 MPa, and at a relatively low strain rate of 8.3 × 10−6 s−1, embrittlement of Ti-6Al-4V materials was not suggested by any of the test results in macro configurations of fractured parts (Fig. 3), micro configurations of fractured surfaces (Section 3.3), mechanical properties of tensile strength and elongation, and reduction of area (Fig. 5), except for a decrease in elongation at 100℃.
Hydride formation16) and the effect of dissolved hydrogen14) are possible mechanisms which cause hydrogen embrittlement in a hydrogen gas environment. In the following paragraphs the test results will be discussed in relation to each of these mechanisms.
4.1.1 Hydride induced HEThe presence of hydrogen in excess of its solubility limit leads to the formation of hydrides. It is important for us to know the solubility limit of hydrogen when we consider hydrides and HE. Numakura's empirical formula for the hydrogen solubility limit in α-phase has been reported17), and this formula would have been of direct use had we been discussing pure titanium, which is entirely in α-phase. No formula, however, is currently available to calculate the hydrogen solubility limit in Ti-6Al-4V, an α+β-phase alloy. Notably, though, the hydrogen solubility limit in α-phase is as low as several tens of ppm18) and the limit in β-phase is extremely high, exceeding several hundreds of ppm19). We therefore carried out computations assuming that any hydrogen solubility limit obtained if our specimens had been entirely α-phase alloy would be smaller than the hydrogen solubility limit in α+β-phase alloy, which is the actual situation. Using Numakura's empirical formula, the hydrogen solubility limit C at the temperature T can be expressed as follows:
| \[{\rm C}({\rm Atom \%}) = 4.7 \times 10^2 \exp \left(- \frac{21.4kJ \cdot mol^{-1}}{RT} \right)\] |
Assuming the supposition that all specimens were in α-phase, the hydrogen solubility limit at 100℃ and 300℃ would be 99 ppm and 1200 ppm, respectively, while the hydrogen concentration at the parallel parts of Ti-6Al-4V specimens tested at 100℃ and 300℃ were 86 ppm and 236 ppm, respectively. Thus, the hydrogen concentrations measured for 100℃ and 300℃ specimens were both lower than the hydrogen solubility limit values based on the assumption that all specimens were in α-phase. As the assumed hydrogen solubility limit values should be smaller than the hydrogen solubility limit values in the actual α+β-phase, the magnitude relationship among the relevant values are expressed as follows: Measured hydrogen concentration < hydrogen solubility limit, based on the assumption that all specimens were in α-phase < hydrogen solubility limit in actual α+β-phase. Ti-6Al-4V specimens formed no or little hydrides in their parallel parts during SSRTs. It is therefore inferred that no HE due to hydrides formation occurred.
4.1.2 HE induced by dissolved hydrogenIn addition to the effect of hydrides12,13), some studies have pointed out the possible effect of dissolved hydrogen14) on titanium, in terms of HE. If dissolved hydrogen causes HE in titanium, it is possible that, as is the case with steel, the stronger a titanium material is, the more susceptible it becomes to embrittlement. Under the conditions of this study, however, we could not detect any HE susceptibility in Ti-6Al-4V, which is high in strength. From this, it is inferred that the effect of dissolved hydrogen on Ti-6Al-4V is insignificant when expressed to hydrogen gas at 100℃ or 300℃ under the pressure of 75 MPa, an anticipated environment in fuel cell vehicles.
4.2 Desorption of hydrogenIn our analyses of hydrogen desorption from the parallel part of Ti-6Al-4V specimens after furacture, we observed the first peak of desorption at 600℃ and the second peak at near 750℃. As the peak hydrogen desorption of pure titanium, which is entirely in α-phase, occurs at 550℃15), the first peak in Ti-6Al-4V, which is in α+β-phase, is considered to have been due to hydrogen from α-phase, and the second peak due to hydrogen from β-phase. The diffusion coefficient of hydrogen is 10−16~10−14 m2/s20,21) in α-phase and 10−12~10−11 m2/s21) in β-phase, showing that hydrogen diffuses faster in β-phase. Combining this with our findings that hydrogen desorption from the first peak increased along with the increase in testing temperature, namely from 100℃ to 300℃, it is inferred that hydrogen diffuses from β-phase and preferentially concentrates in α-phase.
We measured the hydrogen embrittlement (HE) susceptibility of Ti-6Al-4V in a 75 MPa hydrogen gas environment at 100℃ and 300℃ in order to investigate the applicability of pure titanium in equipment which handles hydrogen gas at high temperatures and high pressures. Using a slow strain rate test device which enables testing in hydrogen gas at high temperatures and high pressures, we carried out slow strain rate tests on Ti-6Al-4V specimens at a strain rate of 8.3 × 10−6 s−1. The results obtained are shown below.