2018 Volume 59 Issue 6 Pages 939-943
2018 Volume 59 Issue 6 Pages 939-943
Two sets of 0.1 mol% Sr-doped 5 vol% Ni/Al2O3 and undoped 5 vol% Ni/Al2O3 samples were fabricated by the pulsed electric current sintering technique to investigate the influence of Sr-doping effects on the high-temperature oxidation. Oxidation tests were conducted in air at temperature ranges of 1200–1400°C for 1–10 d. Oxidation of Ni within the matrix at high temperatures induced formations of the top surface layer and the oxidized zone. The top surface layer was composed of the oxidation product-NiAl2O4, while the oxidized zone consisted of Al2O3 matrix and NiAl2O4. Formation of the oxidized zone of undoped and Sr-doped samples followed the parabolic law. The parabolic rate constant of Sr-doped samples was approximately two times smaller than that of undoped samples. The apparent activation energies on the growth of the oxidized zone were determined to be 479 and 476 kJ/mol for undoped and Sr-doped 5Ni/Al2O3, respectively. Sr-doping reduced oxygen transport along Al2O3 grain boundaries and enhanced the high-temperature oxidation resistance of Ni/Al2O3.
Alumina (Al2O3) is one of the most common ceramic materials used in industries with high-temperature structural applications. Among the advantages, such as heat-resistance, high mechanical strength and low-cost production, Al2O3 has the disadvantage of being a brittle material with low fracture toughness due to its strong covalent and ionic bonds. To increase fracture toughness, dispersions of non-oxide phases such as Ni,1) Co2) and SiC3) in an Al2O3 matrix were proposed. As high-temperature structural materials, oxidation of the non-oxide phases may affect many properties of the ceramic-matrix composites. Accordingly, high-temperature oxidation of the ceramic-matrix composites must be intensively studied.
Studies on high temperature oxidation of Al2O3-based composites have been massively carried out over the past few decades. Wang et al.4) investigated the oxidation at 1000–1300°C in air of Ni/Al2O3 composites with 5–15 vol% Ni dispersoid. At high temperatures, oxygen penetrates the oxide matrix, which causes the oxidation of Ni particles within the matrix. Diffusion of oxygen ions and nickel cations induces the formation of a dense NiAl2O4 layer on the top sample surface and an internal-oxidized zone after oxidation. In another study related to high-temperature oxidation of Ni/Al2O3 composites at 1200–1350°C, Nanko et al.5,6) stated that mass transport in the internal-oxidized zone was a rate-controlling process with grain boundary diffusion of oxygen. As a key factor for oxidation at high temperatures of alumina materials, grain boundary diffusion has been subjected to several research works7–10) for understanding their oxidation kinetics.
Several studies have suggested that the segregation of reactive elements such as yttrium,11–13) silicon,14) lanthanum, lutetium and strontium15) could suppress the grain boundary diffusion. For example, the authors’ group previously reported that yttrium12) and silicon14) doping in 5 vol% Ni/Al2O3 composites were effective for decreasing the growth rate of the oxidized zone, which was formed by inward diffusion of oxygen at 1200–1300°C. The reactive elements that have large ionic radii (e.g., Y3+, 0.9 Å; La3+, 1.06 Å; Sr2+, 1.18 Å) may change the grain-boundary environment by “site-blocking”16) and/or strengthening grain boundaries17) because of the localized changes in the bond strength in the vicinity of the dopant ions. Among the reactive elements mentioned above, Sr, which has the largest ionic radii, could be an effective dopant for the suppression of grain boundary diffusion. In addition, strontium is a common addition element used in Al–Si alloys as a eutectic modifier. The addition of strontium in the alloys could affect their oxidation resistance, as well. Accordingly, investigation of the Sr-doping effect on high temperature oxidation of alumina is necessary.
In this study, high-temperature oxidation of 5 vol% Ni dispersed Al2O3 undoped composites (hereafter referred as to undoped 5Ni/Al2O3) and with 0.1 mol% SrO doping (referred as Sr-doped 5Ni/Al2O3) was investigated. With the selected amount of dopant, SrO is attributed to dispersion at Al2O3 grain boundaries. The influence of Sr-doping on high-temperature oxidation of the composites is studied by observation of the formation of oxidized zones on both sets of samples after oxidation in air at 1200–1400°C for 1–10 d.
Fabrication of samples was conducted in the following procedure. A slurry mixture containing Ni(NO3)2·6H2O (Kojundo Chemical Laboratory, purity: 99.9%), Al2O3 (Taimei Chemicals, TM-DAR, d = 140 nm, purity: 99.99%) and distilled water was prepared. Strontium was introduced into the slurry to be 0.1 mol% for the Al2O3 powder by adding a solution containing SrCO3 (Wako Pure Chemical Industries, Ltd., purity: 99.99%) and diluting the HNO3 acid. The slurry was then ball-milled in a plastic bottle with alumina balls for 24 h. The slurry was dropped into a boiling flash that was preheated at 400°C for rapid drying. The dried powder mixture was heat-treated in a stream of Ar–1%H2 gas mixture at 600°C for 12 h to reduce nickel oxide to the metallic nickel. To lessen the agglomeration after the reduction process, the powder mixture was ball-milled in ethanol for 24 h. After drying at 80°C for 12 h, the powder mixture was consolidated with a graphite die by the pulsed electric current sintering technique at a die temperature of 1300°C for 5 min under 50 MPa in vacuum. For comparison, a set of samples without SrO addition was also fabricated using the procedure described above, except that the undoped samples were consolidated at 1200°C for 5 min. The relative density of all fabricated samples attained at least 99% of the theoretical density. The sample surface was ground by a grinding wheel with diamond grains (30 µm) and polished with a diamond particle (2 µm) slurry.
2.2 Oxidation testsOxidation tests of two sets of samples, Sr-doped and undoped 5Ni/Al2O3 were conducted in air at temperatures ranging from 1200 to 1400°C for 1 to 10 d. At the same time, both Sr-doped and undoped samples were put on alumina balls in an alumina crucible and exposed in air at the targeted temperatures with a heating rate of 400°C/h. The oxidizing temperature was monitored using an R-type thermocouple located near the samples. Oxidized samples were then cut to have a cross-sectional view. The cross-sectional surface was polished with the diamond particle slurry. The microstructure of the samples from the cross-sectional view was observed by scanning electron microscope (SEM). For phase identification, samples were subjected to X-ray diffraction (XRD) analysis.
Figure 1 shows SEM images of the fractured surface of undoped and Sr-doped 5Ni/Al2O3 samples. In both sets of samples, the average grain size of Al2O3 was approximately 0.5 µm. Following the solution chemistry routes proposed by Sekino et al.,1) nickel particles that have an average particle size less than 100 nm in diameter were dispersed homogeneously at the matrix grain boundaries. No strontium oxide or related products were detected by XRD because of their low concertation (0.1 mol%).

SEM images of the fractured surface of (a) undoped 5Ni/Al2O3 and (b) Sr-doped 5Ni/Al2O3 samples.
Figure 2 shows SEM images of the two samples observed on cross-sectional surface after oxidation at 1300°C for 1 d in air. The Al2O3 matrix can be seen as the dark color phase over the samples. Metallic Ni particles as the bright dots were observed in the region below the oxidation front. In the oxidized zone, only two phases (the dark and gray colors) were identified. No metallic Ni particle was found in the oxidized zone. These facts indicated that the metallic Ni particles were completely oxidized in that region. The oxidized zone included only the Al2O3 matrix and the oxidation product. In addition, voids were observed in the oxidized zone. Accordingly, the oxidized zone was defined as the region ranging from the top surface to the oxidation front. On the top surface, formation of a layer that has the same color as the oxidation product was noticed in the oxidized zone. Based on the results of XRD analysis as shown in Fig. 3, NiAl2O4 was the only oxidation product. NiO does not coexist with Al2O3, which has been discussed elsewhere.5,8,12,14) This fact obviously indicates that the top surface layer is the NiAl2O4 layer. Noticeably, the thickness of the top surface layer observed on an undoped sample was slightly higher than that of a Sr-doped one. This is in good agreement with the XRD results in which, the intensity of NiAl2O4 signals of undoped samples seems to be higher than that of Sr-doped samples. Thus, the thickness of the oxidized zone formed on the Sr-doped sample was smaller than that of undoped sample after oxidation under the same conditions.
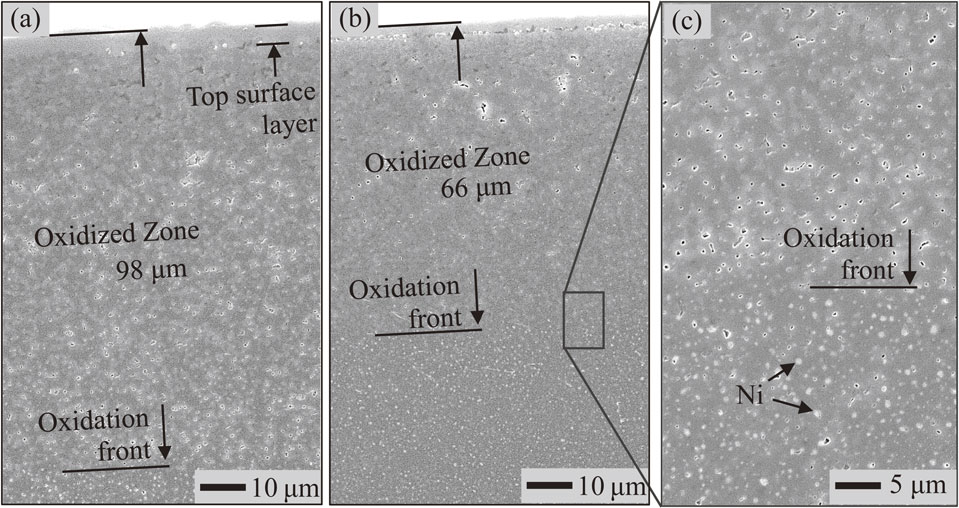
SEM images of cross-sectional view observed on (a) undoped 5Ni/Al2O3, (b) Sr-doped 5Ni/Al2O3 sample after exposition to air at 1300°C for 1 day and (c) higher magnification image of the region includes the oxidation front.

XRD patterns of undoped 5Ni/Al2O3 and Sr-doped 5Ni/Al2O3 samples after oxidation in air at 1300°C for 1 day.
Figure 4 shows the thickness of the oxidized zone versus oxidation time at different temperatures for undoped and Sr-doped 5Ni/Al2O3 samples. Although there are some deviations in the formation of the oxidized zone at 1400°C, no significant difference in the microstructure between the sample oxidized at 1400°C and samples oxidized at the other temperatures was found. However, these deviations observed in the undoped and Sr-doped samples were very similar. The deviations were thus attributed to errors in the experimental procedure. Results of the investigation indicated that the thickness of the oxidized zone formed in the Sr-doped samples were smaller than that of the undoped samples at all the tested temperatures. Growth of the oxidized zone follows the parabolic law and can be described by the following equation:
| \begin{equation} x^{2} = k_{\text{p}}t, \end{equation} | (1) |
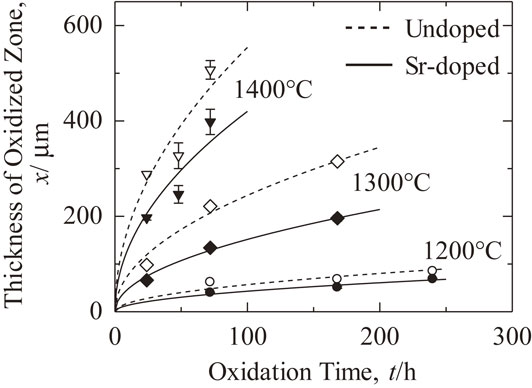
Thickness of oxidized zone as a function of oxidation time at various oxidizing temperatures in air.
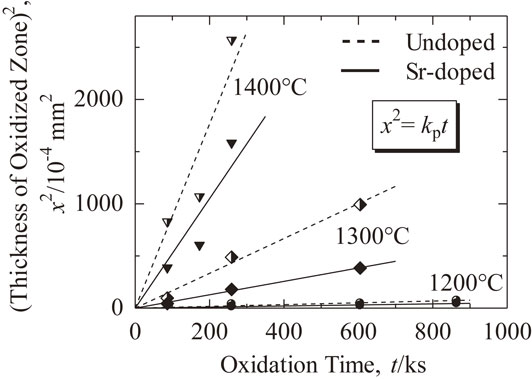
The square of the thickness of the oxidized zone (x2) versus oxidation time (t) at different oxidation temperatures for undoped 5Ni/Al2O3 and Sr-doped 5Ni/Al2O3.
Table 1 shows a comparison of the values of parabolic rate constants at different temperatures for undoped and Sr-doped samples. In all cases, the values of parabolic rate constants for undoped samples were higher (about two times) than that of Sr-doped samples. In other words, the rate constants of Sr-doped samples were reduced by a factor of about 2 compared with that of undoped samples.

Figure 6 plots the common logarithm of the parabolic rate constants versus the reciprocal of oxidation temperatures for undoped and Sr-doped 5Ni/Al2O3, which display the Arrhenius behavior. The apparent activation energies for the growth of the oxidized zone as calculated from the Arrhenius plots for undoped and Sr-doped samples were determined to be 479 ± 10 and 476 ± 10 kJ/mol, respectively. The parabolic rate constants for undoped and Sr-doped samples are correspondingly given by following equations:
| \begin{equation} k_{\text{p}}(\text{Un})/\text{m$^{2}\,$s$^{-1}$} = 946\times\exp\frac{-479\,\text{kJ$\,$mol$^{-1}$}}{RT}, \end{equation} | (2) |
| \begin{equation} k_{\text{p}}(\text{Sr})/\text{m$^{2}$s$^{-1}$} = 385\times \exp\frac{-476\,\text{kJ$\,$mol$^{-1}$}}{RT}. \end{equation} | (3) |
At high temperatures, oxidation of nickel particles within the matrix induced the formations of a NiAl2O4 top surface layer and oxidized zone. The driving force for those formations is the difference in oxygen potential between the surrounding atmosphere and the oxidation front. The difference in chemical potential of oxygen gives a difference in chemical potential of nickel and aluminum, which leads to the diffusion of oxygen ions, nickel and aluminum cations along grain boundaries during oxidation. The oxidation behavior mainly depends on the volume fraction of Ni dispersion, impurity/doping elements and grain size of alumina matrix/nickel particles.
The value of the apparent activation energy for undoped 5Ni/Al2O3 in this study (479 kJ/mol) was slightly higher than that of the undoped 5Ni/Al2O38) (400 kJ/mol) investigated at lower temperatures by the authors’ group previously. At higher oxidation temperatures, Al2O3 grains could become coarse, as shown in Fig. 7. The coarsening of Al2O3 grains reduced the amount of grain boundaries, which decreased the flux of oxygen diffusion along Al2O3 grain boundaries.8) This means that Ni/Al2O3 composites with a microstructure at a submicron scale might have an accelerated oxidation rate at a lower temperature and shorter oxidation time. As this study investigated oxidation at higher temperatures and longer oxidation times, the dynamic grain growth had stronger influences on the oxidation rate. Therefore, the value of activation energy derived in the previous study was slightly lower than that derived in this study.
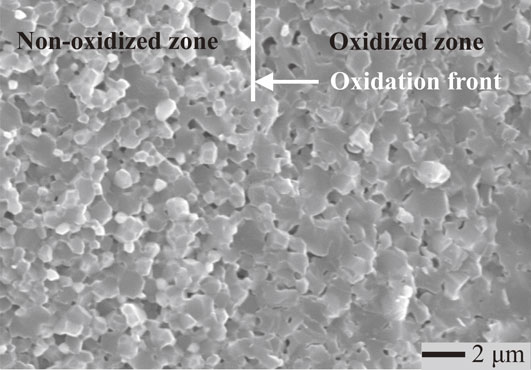
SEM image of fractured surface of undoped 5Ni/Al2O3 after heat-treatment at 1300°C for 12 h in air, showing the coarsened grains in both the oxidized and non-oxidized zones after oxidation.
As shown in Fig. 6, the slopes of the lines representative for undoped and Sr-doped samples are very similar. It implies that Sr-doping does not cause any drastic change in the apparent activation energy. Unlike Sr-doping, the apparent activation energies in cases of Y-doping and Si-doping seem to have larger values than those of the undoped samples. Table 2 shows a comparison of the calculated values of the apparent activation energies from oxidation experiments of Ni/Al2O3 composites in terms of doping elements, the volume fraction of Ni and grain size of the Al2O3 matrix. The values of the activation energies of undoped samples range from 400 to 550 kJ/mol depending on the nickel content, grain size of Al2O3 and investigated temperatures. Meanwhile, the activation energies of Y-doped and Si-doped Ni/Al2O3 with 5 vol% Ni have larger values than that of Y-doped Ni/Al2O3 with 0.5 vol% Ni. For example, Nanko et al.12,14) reported that the activation energies of Y- and Si-doped 5 vol% Ni/Al2O3 have considerably larger values than that of undoped samples. With a lower volume content of Ni (0.5 vol%) in the study reported by Cheng et al.,11) the influence of NiO co-doping seemed to be mitigated. Accordingly, the values of the activation energies of undoped and Y-doped 0.5 vol% Ni/Al2O3 were comparable. This means that Y and Si doping in 5 vol% Ni dispersed Al2O3 is less effective at suppressing grain-boundary diffusion at temperatures above 1350°C. It was suggested that the existence of NiAl2O4 in the oxidized zone could decrease the effects of Y and Si doping owing to the co-doping with NiO at grain boundaries of the matrix.

The thickness of the top surface layer observed on undoped sample was slightly higher than that of Sr-doped one. Formation of the top surface layer was a result of cation diffusion (mainly Ni2+). Because the Sr-doping affects the oxygen diffusion, it affects the diffusion of cations as well. As a result of the Sr-doping effect, the development of the top surface layer and the oxidized zone was slower than that of undoped sample.
The results of the present study indicate that Sr-doping is effective at suppressing the grain-boundary diffusion of oxygen at 1200–1400°C. Sr-doping is effective at reducing the parabolic rate constant by a factor of 2. Sr-doping shows less influence on the activation energy of oxygen diffusion, which is different from Y- or Si-doping. In summary, Sr-doping enhances the high-temperature oxidation resistance of Ni/Al2O3, even with a high-volume content of Ni.
The influence of Sr-doping effects on high-temperature oxidation of 5 vol% Ni/Al2O3 was investigated at a temperature range of 1200–1400°C in air. Oxidation of Ni within the matrix at high temperatures induced formations of the top surface layer and the oxidized zone. The top surface layer was composed of the oxidation product, NiAl2O4, while the oxidized zone consisted of the Al2O3 matrix and NiAl2O4. Formation of the oxidized zone of undoped and Sr-doped samples followed the parabolic law. The parabolic rate constants of Sr-doped samples were about two times smaller than that of the undoped samples. Sr-doping into the Al2O3 matrix was effective at reducing the formation of the oxidized zone at the investigated temperatures. In other words, Sr-doping demonstrated a beneficial influence on oxygen transport along Al2O3 grain boundaries.
The authors wish to express their appreciation to the Japan Science and Technology Agency for providing partial financial support for this study in the frame of the Advanced Low Carbon Technology Research and Development Program.