2019 Volume 60 Issue 7 Pages 1356-1366
2019 Volume 60 Issue 7 Pages 1356-1366
Recent years have witnessed a series of numerous investigative activities to improve existing metallic biomaterials (Ti and Ti alloys, stainless steels, Mg and Fe alloys) by their nanostructuring for advanced medical applications using severe plastic deformation (SPD) processing. Nanostructured metals are peculiar for their enhanced strength and fatigue life, which makes them an excellent choice for fabrication of implants with improved design for dentistry and orthopedics. Moreover, surface modification of nanometals by chemical etching and bioactive coatings show a significant improvement of biomedical properties. Various studies conducted in this field make it possible to fabricate miniaturized dental implants and nanoTi plates with enhanced osseointegration.
R&D approach for manufacturing of medical implants with improved design and biofunctionality using nanostructured metals and their surface modifications
Presently, over 70% of implant devices are constructed from metallic materials because of their strength, toughness and durability.1) On the other hand, another principal concern in the use of metals in the human body is their safety in terms of toxicity with metal ion release, which in turn justifies the application of corrosion-resistance materials, mostly such as stainless steel, Co–Cr–Mo alloy, CP Ti and Ti alloys, and more recently - bioresorbable Mg and Fe alloys. However, to have good biofunctionality, metals should be improved with additional properties (electrical properties, wear resistance, bioactivity and so on) before they can be used for medical devices.1,2)
New alloys with optimized chemical composition, manufacturing processes and surface modifications undergo continuous research and development to satisfy the clinical demands for medical devices.2,3) At the same time, during the last two decades, the nanostructuring of metals to improve their properties using the so-called «severe plastic deformation» (SPD) processing has become a new and promising area of modern materials science and engineering.4,5)
Nanostructuring of metallic materials by various SPD techniques comprises grain refinement of the microstructure down to submicron or nanosized range as well as the formation of nanoclusters and nanoprecipitates of secondary phase, which essentially influences the mechanical and functional properties of the materials.5,6) Up to now a whole variety of SPD techniques have been introduced and developed to provide heavy strains (ε > 5–7) under high applied pressure including multiple forging, accumulative roll bonding (ARB), twist extrusion and other (see, e.g., the recent monographs and reviews.7–9) However, the most popular techniques even today are high pressure torsion (HPT) and equal channel angular pressing used for the manufacture of ultrafine-grained materials and put forward already in pioneer works.4) Recently, the techniques have been further developed for practical use.10,11)
From the perspective of medical applications, the use of SPD processing techniques for the formation of nanostructures in metals and alloys may positively affect both mechanical and biomedical properties.12) For the latter, surface modification of bulk nanomaterials also plays an important role.13,14) All of this provides the possibility for the development of medical devices with improved design and functionality. This article describes the progress in recent studies of such relevant issues.
The first works in this regard were applied to commercially pure titanium (CP Ti) because of its highest biocompatibility with living tissues among various metals,15) and this was the focus of many clinical studies of medical tool and devices applied in trauma, orthopedic and dental practice. But unlike other metallic materials used in biomedical devices, CP Ti has somewhat low strength properties. Typically, with higher strength levels resulted from either alloying or secondary processing, the materials usually lose biometric response and fatigue behavior. Then, an alternative strategy was developed to prove that nanostructuring CP Ti by SPD processing may become a novel and high-performance approach to enhance the mechanical properties of this material to the next level.16–20) This strategy also has the advantage of improving the biological response of the CP titanium surface.21)
The earliest results on nanostructured CP Grade 4 Ti [C – 0.052%, O2 – 0.34%, Fe – 0.3%, N – 0.015%, Ti-bal. (wt. pct.)] were performed by Valiev et al. with the aim to develop the material in the form of long-length rods with superior mechanical and biomedical properties for the fabrication of dental implants.22) Processing involved SPD technique by equal-channel angular pressing23) and further thermo-mechanical treatment (TMT) through forging and drawing to improve the nanostructuring of 7-mm diameter and 3-m long bars. After combined SPD and TMT processing a large reduction in grain size was observed, in particular from the 25 µm in the initial Ti rods to 150 nm of the processed one, as in Fig. 1. The selected area electron diffraction (SAED) pattern, Fig. 1(c), illustrates a typical for nanoSPD processed materials picture when the grain boundaries in ultrafine grains are of predominantly high-angle type with increased internal stresses.4)

Microstructure of Grade 4 CP Ti before and after SPD processing: (a) the initial rod; (b), (c) after ECAP and TMT (Optical and electron micrographs).
Rods of CP Ti produced by a continuous SPD method, known as ECAP-Conform, followed by drawing to produce the necessary length had a similar structure.20,24) Here, the main issue was to provide the formation of homogeneous ultrafine-grained structure along the entire three-meter rod lengths for economical fabrication of implants and to develop the SPD technology for mass production of nanoTi.
Table 1 illustrates the advantages of mechanical properties in CP titanium after nanostructuring by ECAP and TMT. As is seen, the nanostructured titanium has 2 times higher strength than the conventional CP titanium without any drastic ductility reductions (to below 10% elongation to failure) normally observed after rolling or drawing.
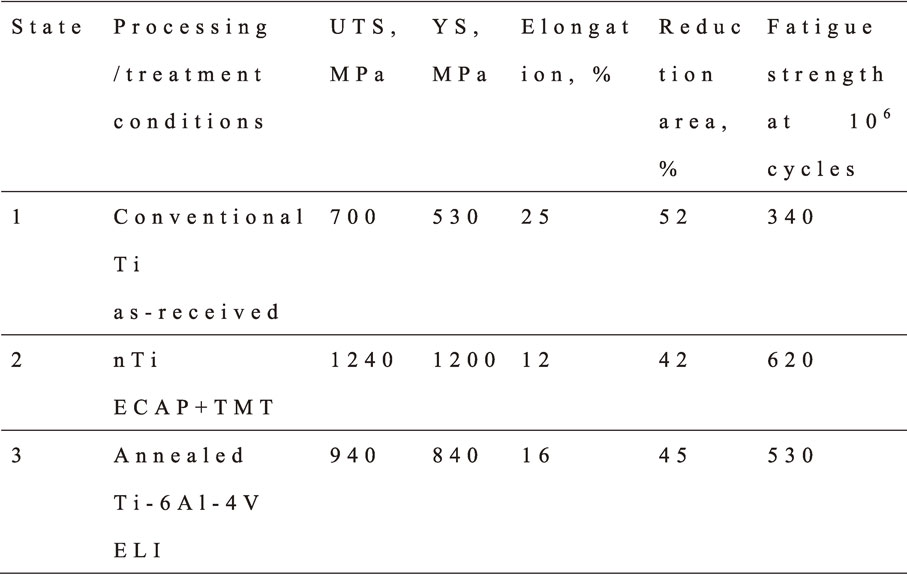
Fatigue testing for nanostructured and conventional CP titanium conducted at room temperature in air was performed per ASTM E 466-96 at a load ratio R (rmin/rmax) = 0.1 and loading frequency of 20 Hz. As shown in Table 1, the fatigue strength of nanostructured CP titanium after one million cycles is about two times higher than that of conventional CP titanium and exceeds that of the Ti–6Al–4V alloy.15,25) Considerable improvement of fatigue properties was also shown in the rods from nanostructured Ti Grade 4 produced by ECAP-Conform and drawing.13,24) The high strength of nanoTi allows development of smaller sizes of implants, which reduces the level of surgical intervention (see, also, Section 3).
Recent studies have shown that the nanostructured Ti after SPD demonstrates the improvement of biological reaction on the surface as well. This was confirmed in a series of experiments through cytocompatibility tests using mouse fibroblast tests.26–30)
Titanium is known for its very high biocompatibility as a result of the protective oxide film, or titanium dioxide, TiO2, that forms naturally on its surface and provides a stable surface on which a mineralized bone matrix can be set. This film is usually 50–100 Å thick and biologically inert, thus it prevents reaction between the metal and the surrounding body environment.15)
The presence of ultrafine-grained structure and non-equilibrium strain-distorted grain boundaries in nanoTi produced by SPD significantly increases the internal energy of the material4) and, as a result, this may considerably change the morphology of oxide film. This might be due to the fact that processing by SPD is typically favorable for cellular attachment to titanium with polished surfaces.21) At the same time, surface modification opens even more possibilities for improvement of biomedical properties of nanostructured titanium (see Section 4).
2.2 Titanium alloysCurrently among commonly used metallic materials in the dental and orthopedic fields, titanium alloys such as Ti–6Al–4V (Ti64) and Ti–6Al–7Nb continue to be the most important components due to their high mechanical properties and relatively good biocompatibility.15,31–34) However, aluminum and especially, vanadium are rather toxic elements and further reducing of the Young’s modulus of these alloys is also a relevant challenge for today. Thus, there is a reasonable necessity to develop a new generation of titanium alloys with improved strength, lower Young’s modulus and better biocompatibility compared to Ti64.2,3) Close attention is paid to optimization of titanium alloying, in particular for the systems Ti–Nb and Ti–Mo. At the same time, one of the new approaches that may play a key role to provide these improvements is based on nanostructuring of titanium alloys by SPD techniques.12)
Recent studies progressively contributed to increasing mechanical and functional properties of titanium alloys using their nanostructuring. Key results are described below.
In Refs.35–37) complex studies of the microstructure and mechanical properties of Ti–6Al–4V ELI (extra low interstitial alloys for medical applications) processed by SPD were conducted. The alloy was in a form of round rods 40 mm in diameter (Intrinsic Devices Company USA) and with chemical composition as following: Ti – base, Al – 6.0%; V – 4.2%; Fe – 0.2%; C – 0.001%; O2 – 0.11%; N2 – 0.0025%; H2 – 0.002% (mass%). The two-phase alloy had the following microstructure in the as-received state: a grain size of about 8 µm in a cross-section, 20 µm in a longitudinal section. According to the X-ray analysis, the corresponding volume fractions of α and β phases was about 85% and 15%, respectively. The processing of the 250-mm length rods was divided into two steps. At first, the rods were subjected to ECAP at 600°C via route Bc and then further extrusion with total strain 4.237) to produce the rods 18 mm in diameter and up to 300 mm in length. The extrusion was carried out at 300°C with the last pass at room temperature for additional strengthening. Further treatment included annealing in the range of temperatures from 200°C to 800°C for 1 hour and subsequent cooling in air.
TEM studies revealed that SPD intensifies grain refinement and leads to a complex UFG structure forming of the grains and subgrains with a mean size of about 300 nm.
Figure 2 displays typical stress–strain curves for the CG and UFG alloy demonstrating that the alloy after grain refinement by SPD undergoes considerable strengthening. Tensile elongation of the UFG alloy (curve 2) is reduced from 17% down to 9% in contrast to the material in the as-received state (curve 1). Figure 2 shows that strength and ductility further improved (up to 12%) from subsequent annealing at 500°C, with uniform elongation of about 4%. The results of tensile tests correspond accurately to the microhardness measurement data (Fig. 3).

Engineering stress–strain tensile curves of the Ti–6Al–4V ELI alloy: initial, coarse-grained state (1); UFG state before (2) and after annealing at 500°C (3).

Influence of annealing temperature on microhardness of the UFG Ti–6Al–4V ELI alloy; annealing time is 1 h.
In accordance with Ref.37), ductility enhancement in the UFG alloy after annealing is obviously associated with such factors as decrease in internal elastic stresses and dislocation density. At the same time, the observed decay of metastable β-phase during cooling from the annealing temperature explains additional strengthening of the alloy. Its volume fraction in the structure of the UFG alloy at 500°C can be higher than before annealing, as has been shown in Ref.38), using quenching from the annealing temperature. Though there are no visible particles of any second phase, aging processes could cause the formation of grain boundary segregations that may additionally increase the properties of the UFG alloy after annealing.39,40)
Investigations of fatigue properties of the Ti–6Al–4V ELI alloy with ultrafine grains revealed that high strength and enhanced ductility after SPD processing and additional annealing at 500°C (1370 MPa and 12%) resulted in fatigue limit enhancement on the basis of 107 cycles up to 740 MPa in comparison with 600 MPa in the initial coarse-grained (CG) state (Fig. 4).

Fatigue test results of the samples made of CG and UFG alloy after annealing at 500°C, 1 hour.
The fatigue limit for the UFG Ti–6Al–4V alloy reported in Ref.35) under the conditions of rotating bending was only slightly higher than the earlier value in Refs.36,41), which proves the fact that the level of fatigue properties may depend on the measurement technique.
Thus, the results show that high strength can be achieved in UFG Ti–6Al–4V ELI alloy through ECAP and additional mechanical and thermal treatment. Moreover, it is possible to manipulate the grain boundary structure and phase morphology in the UFG alloy by changing the SPD regimes and particularly, such processing parameters as temperature, strain rate, strain, and consequently achieve the best combinations of strength and ductility, as well as the improved fatigue endurance limit of up to 740 MPa, well beyond the 600 MPa level measured in the coarse-grained alloy.
Another recent work42) is devoted to enhancement of strength and ductility of the Ti–6Al–7Nb alloy. If compared to Ti–6Al–4V, the alloy is a better choice material for human body as it is less harmful. In addition it demonstrated rather attractive properties after processing by ECAP with thermo-mechanical treatment when the formation of UFG structure led to higher strength (UTS = 1400 MPa) and ductility (elongation 10%). These levels of properties are very promising for the new design and fabrication of high-performance medical implants.
As indicated above, recent interest has been drawn to titanium alloys based on Ti–Mo and Ti–Nb systems with mainly the β phase because the alloys have Young’s moduli in the range of 55–90 GPa, and thus possess lower stress shielding.43–47) Plus, these Ti alloys contain only non-toxic elements such as Nb, Mo, Zr, and Ta. However, the single phase β-Ti alloys that display the lowest Young’s modulus are commonly obtained after solution treatment and thus are relatively soft. An ideal combination would be high strength and low Young’s modulus, which is hardly true for these materials. Substantial strengthening can be achieved by ageing treatments that induce a fine and uniform precipitation of ω and α phase components, but this inevitably increases the Young’s modulus of the alloy.43,44,48,49) Advancements in the areas of dentistry and orthopedics called for new strategies to develop β-Ti alloys with low Young’s modulus and high strength that are more suitable for such applications.
Recent developments deal with introducing SPD processing as a promising way to fabricate nanocrystalline β-Ti alloys that should have simultaneously high strength, low modulus of elasticity and excellent biocompatibility.50–54) Nanostructuring of the alloys leads to such desirable mechanical properties as higher strength resulting from grain refinement and substructure55) and lower rigidity arising from the complete removal of the ω phase and nanocrystalline structure, plus surface modification contributes to improved biological responses. The nanocrystalline β-Ti alloys also display excellent in vitro biocompatibility, shown by enhanced cell attachment and proliferation.56) These novel nanocrystalline β-Ti alloys have high chances to meet the challenge of next-generation implant material with significant prospects in load bearing biomedical applications.
2.3 Stainless steelsAustenitic stainless steels are also the most widely used medical biomaterial due to excellent corrosion and oxidation resistance and good formability. However, low mechanical strength and poor anti-friction properties of these materials are the critical obstacles for their application. In the last two decades much attention has been drawn to strengthening the stainless steels and several approaches have been developed, including SPD processing.12)
As was said, austenitic stainless steels can be strengthened by severe plastic deformation. For example, the strength increase from 515 MPa to 1647 MPa was demonstrated on 316L stainless steel in Ref.57). Similarly, a yield strength of 1460 MPa in a duplex 32304 stainless steel after just 4 ECAP passes was shown in Ref.58). Moreover, as was presented,59) the nanostructured SPD 316L steel exhibited extremely high yield strength up to 2230 MPa, which is the highest ever reported value in literature for austenitic steels.
Plastic deformation has become an effective method to process stainless steel that are quite responsive to SPD, however the implementation of the SPD-processed stainless steels in practice has been slow. This is partially due to the fact that SPD causes apparent changes in microstructure of stainless steels that need to be more accurately controlled. SPD readjusts the phase compositions from those normally expected in stainless steel. This occurs through mechanisms including stress-induced and strain-induced phase transformation, including the martensite formation. For example, strain-induced martensite was nucleated during ECAP of 30160) and 304 stainless steels.61) SPD introduces nanostructural features such as nano-twins, micro-shear bands, very high dislocation densities and substructures. SPD also changes the formation and distribution of carbides. These nanostructural features may significantly influence on the annealing and recrystallization behaviors of stainless steels and therefore their surface properties.62,63)
On the other hand, the surfaces of nanostructured stainless steel provide enhanced corrosion resistance64,65) and cell growth and proliferation.66,67) These results are encouraging indicators of the potential for nanostructured stainless steels for biomedical applications.
2.4 Magnesium alloysMagnesium is a biodegradable and bioresorbable material with very light weight and, therefore, it is widely tested as a potential implant material.68–71) As the lightest of all light metal alloys used for structural applications (except beryllium) magnesium is an excellent choice to be used for engineering of medical devices when weight is a critical design matter and the range of such applications may vary from wheelchairs and stretchers to surgical tools, vascular stents and orthopedic implants.71–73) However, the following four major limitations must be addressed for the traditional Mg alloys: limited strength, ductility, corrosion resistance and highly anisotropic mechanical properties. But all four of these limitations may be addressed mostly through nanostructuring by SPD processing.12,74,75)
The most interesting aspect of recent studies on improving the properties of magnesium alloys by SPD processing regards modifying their strength, ductility, and corrosion behavior to satisfy the requirements for biomedical applications.71,75–78) The prospect of bioresorbable magnesium implants has gained significant attention in the medical community, and has become the focus of extensive reviews.12,74,79)
Magnesium is a suitable candidate for stent applications but pure metal is rarely used due to its low corrosion resistance. Nanostructuring of Mg-based alloys offers a variety of alternative ways and solutions to alloying for design and manufacturing of a magnesium stent. First, the corrosion rates are subject to change depending on grain size reduction. Hao et al.80) revealed that the corrosion rate in Hank’s solution can be reduced by subjecting an AZ31 alloy to ECAP, however the obtained values were still not suitable for stent applications. Hadzima et al.81) demonstrated improved electrochemical properties in the AZ80 alloy after combined processing by ECAP and extrusion due to the formation of an ultrafine grain structure; thus it became possible to produce the polarization layers that remained stable and resisted degradation up to 96 hours. Most recent studies by Minárik et al.82) were focused on testing the electrochemical characteristics of AE21 and AE42 alloys after 8 passes of ECAP. As a result, the grain size was reduced, thus increasing the corrosion rate in the alloy AE21 owing to enhanced chemical activity at the grain boundaries. In contrast, the corrosion rate in the alloy AE42 after similar processing by ECAP was reduced. In the latter case, the effect of a smaller grain size was overruled by the larger effect of increased uniformity of the spatial distribution of alloying elements. Obviously, nanostructuring of magnesium alloy for stent application is a complex task, when the effects of processing on every particular alloy should be carefully considered, which makes it even more challenging and attractive for further studies.
Significant improvement of strength and ductility of magnesium alloys by nanostructuring provides better formability during stent processing and increases the in situ expandability and the force bearing capability of the inserted nanostructured magnesium stents. A very high level of properties for bioabsorbable magnesium alloys have been reported by Kutniy et al.83) and Dobatkin et al.75) for alloy WE43 and by Pachla et al.84) for AZ31, AZ 61, and AZ91 alloys. It is necessary to outline and achieve suitable combinations of mechanical properties and for this overall stent design factors that influence stent-host interactions should be considered.
The superior mechanical properties of nanostructured metals enable the development of improved design for medical implants. Recently, we have studied two examples of making and using these implants from nanoTi with premium design for dentistry and orthopedics.13)
3.1 Nano Ti miniplatesChange of materials, such as the case with substituting nanostructured CP Ti for CG Ti, leads to altering the design of devices and this is when a set of simple rules should be taken in account. Lately, the necessary calculations were conducted to analyze the geometry of miniplates made of nanostructured Ti for maxillofacial surgery.13,85)
A mini-plate made of CP Ti, as specified by ASTM F 67, was used by a “Conmet” company (Moscow, Russia) as the starting point for redesigning the product dimensions for making a nanostructured CP Ti mini-plate. The cross-section properties of a new plate were computed using the estimates of the fatigue endurance limit for coarse-grained Grade 4 Ti σf(CG) and nanostructured Grade 4 Ti σf(NS).
Comparison of bending strength for mini-plates from conventional CG Ti and nanostructured Ti was performed86,87) because plates commonly support bending loads.15) Further, the fatigue strength of mini-plates was compared through testing with an ElectroPuls E3000 system.87) The number of cycles to failure N was determined through cyclic 3 mm displacements at 30 Hz. Triplicate tests were completed.
Table 2 provides the estimation results for cross-section area of the nano-Ti plate.85) The width and the diameter remain the same as in the standard item, whereas the plate thickness alters from 0.9 to 0.7 (Fig. 5).


Image of a mini plate with six holes made of nanostructured Grade 4 Ti.
These experiments showed that the plate strength does not necessarily decreases in the process of bending even when the cross-section area is reduced.
Bending tests were completed for the experimental values of the fatigue life of plates, as is shown in Fig. 6. The standard plates have sustained 17000 ± 500 cycles and the redesigned plate made from nanostructured Ti could survive a larger number of cycles to failure (105000 ± 800). This result indicates that the plate from nano-Grade 4 Ti has its bending strength significantly improved and thus, speaks of its clear advantage over the standard item manufactured from coarse-grained Ti.

Fatigue life of the standard plate and redesigned plate from nano-Ti.
Numerous nanoimplant® dental implants have been produced from pure nanostructured Grade 4 Ti rods processed by the ECAP-Conform on CNC machines in diameters of 2.0 mm, 2.4 mm and 3.5 mm with the intraosseal part length of 8, 10, 12 and 14 mm by the company “Timplant” s.r.o., Czech Republic over the last years (http://www.timplant.cz/en/) (see also Ref. 13)). The implants are characterized by an etched threaded tapered intraosseal part with a polished gingival part and prosthetics cone top with interior thread above it, and with anti-rotation crown element. High primary stability is achieved by an enhanced thread design with self-drilling thread groove. Special etching provides the surface roughness (see Section 4 for details).
Nano-CP Ti is responsible for having no toxic alloying elements (such as V or Al) or allergens (such as Ni, Co, or Cr) and it is clearly attractive for the production of thin implants owing to its high strength. Statistical observation completed over two years by five surgeon dentists both from state-owned and privately-run dental surgeries in the Czech Republic88) demonstrated high clinical performance of 2.4 mm Nanoimplants®.
Surface properties are critical for medical implants and their modification has proved to accelerate osseointegration. In particular, pure Ti is bionert material meaning that when placed in the human body it has minimal interaction with surrounding tissue, including the human bone.20) Application of SPD processing to achieve nano-sized grains in CP Ti allows various types of bone-forming cells to adhere and proliferate with better efficiency and this was demonstrated in numerous studies.20,30,89–91) Additional surface modification may further contribute to better bioactive performance of implants made of nano Ti. Recently, two main approaches gained significant interest in dental implant surface modification: chemical etching and deposition of bioactive coatings.13,92–98)
The topography of etched surfaces is strongly determined by etching solution and etching time. Different solutions, such as acidic (H2SO4/H2O2) or basic (NH4OH/H2O2) Piranha solutions can be used for etching of CP Ti97) resulting in different surface topographies. The surface topography was found to be clearly modified after varying etching time and the effect was more pronounced in the nano Ti, as it has been very recently demonstrated in Fig. 7.97)

Surface of CG and NS samples of Grade 4 Ti after mechanical polishing and etching in the mixture of acids 30% HNO3 + 3% HF+H2O for 20 minutes: (a) CG Ti surface; (b) nano-Ti surface. SEM images.
Biocompatible coatings can also efficiently make the nano Ti implants integrate with the human bone.95,96) Presently, the research into synthesis of biocompatible coatings integrating the inorganic (Ca-, P- containing phases) and organic (biologically active and bioinert molecules) components on titanium implants appears to be topical state of the art.99,100)
Plasma electrolytic oxidation (PEO) offers excellent possibilities for developing Ca-, P- containing biocompatible coatings on the surface of implants both for orthopedics and traumatology applications.101,102) The majority of implant materials, both conventional as titanium, and prospective as magnesium and zirconium, belong to a group of so-called valve metals that can be easily coated with the PEO technique.103,104) Currently, PEO is extensively researched for development of biocompatible and bioactive coatings on Ti, Mg, Zr alloys, including nanostructured.13,98,105,106) The PEO process is an expansion of the traditional anodizing into the high voltages up to 600 V; these voltages promote microdischarges within the coating; this results in its resolidifying and intensive growth.107–109) The coatings obtained by this method contain stable titania (rutile and anatase) tightly attached to the surface because of the process mechanism which includes electrochemical oxidation and numerous melting and crystallizing events at the microdischarge sites (Fig. 8(a)).110,111) This mechanism provides the surface morphology with regulated roughness and porosity (Fig. 8(b), (c)) welcoming the osteoblast adhesion in the human body.112,113) As it was shown recently, nano Ti coated with the PEO technique has finer structure, contains more biocompatible compounds and shows higher cell adhesion compared to the coating on CG Ti.98)
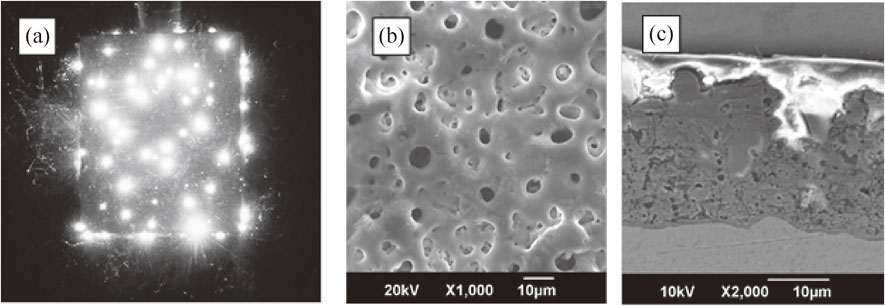
Microdischarge appearance during PEO (a); PEO biocompatible coating on nanostructured Ti, top view (b) and cross-section (c).
For the orthopedic applications, the PEO coating opens possibilities to develop an “ideal coating”. This coating provides enhanced biocompatibility due to following the biomimetic approach at physical, chemical and biological levels.98,114) At physical level, a gradual change of the elasticity modulus from the metallic implant to the bone is achieved because the wide network of the pores forms a fractal-like structure, with the pore size increasing to the top of the coating.112) Unlike other methods, for the PEO there is no need to create a surface with high roughness.101,115) The high surface area of the porous PEO coating promotes osteoblast attachment on the implant surface,116) because the sponge-like coating morphology exhibits the surface roughness (Ra) from 1 to 5 µm and rounded pores with a diameter from 0.1 to 10 µm; this provides the sites for mechanical attachment of the cells with protein pseudopodia.117,118) Adhesion of the PEO coatings is higher than that of the other coating types.119) At the chemical level, by applying varying pulse polarity during PEO, it is possible to incorporate the anions and cations of the electrolyte into the coating; this provides Ca- and P- containing bioactive crystalline phases within the coating: hydroxyapatite, tricalcium phosphate, tetra calcium phosphate, perovskite. Since the early works,120) the PEO coating technology for implant materials has been significantly developed so that now these coatings incorporate species like silver providing anti-bacterial response and compounds like hydroxyapatite stimulating the bone growth.116,121) At the biological level, the biomimetic properties can be introduced by applying a bioactive organic top coat into the PEO pores. For example, peptide LL-37 in combination with phospholipids used with PEO coatings helped to achieve antimicrobial activity for titanium implants.122) A recent study of nanostructured titanium subjected to PEO and further modification with RGD containing peptide showed significant improvement of all the crucial implant properties: strength, corrosion resistance and biocompatibility.98) Therefore, the research into the development of the biocompatible coatings on nanostructured implants by PEO is an actual problem of the modern engineering and technologies.
Further advances in the PEO treatments to develop a biomimetic coating can be summarized as shown in Fig. 9. This includes changes in electrical regimes, electrolyte composition, and applying post treatments. As follows from specific studies, pulsed bipolar treatment does not necessarily provide the best protective properties for the coatings. This concerns Mg and Zr.123,124) Also, the electrolyte optimization is also not a straightforward technique. For example, Mg PEO in early studies was performed in fluoride containing electrolytes;125,126) this is not an environmentally friendly approach. Currently, the non-fluoride electrolyte was proposed.127) One of the major drawbacks of these electrolytes is the application of very low soluble components, e.g. Ca3PO4 or Ca(OH)2; this makes the electrolytes cloudy and unstable. Therefore, using complex forming agents, e.g. EDTA, transfers the electrolytes into colloids with higher stability.113,128) An important aspect of the PEO coating bioactivity increase is the development of higher hydroxyapatite (HAp) content. Usually, Ca-, P- containing phases in the PEO coating have a various crystalline structure, and also a significant amount of amorphous phase is present. However, application of a hydrothermal post-treatment provides recrystallization of the amorphous phase and formation of the HAp crystallites.119) Electrophoretic deposition is another surface modification method which can be coupled with the PEO to form a duplex process. The mechanism of electrophoresis coupled with the PEO can help to enrich the coating with the nanoparticles that are pre-charged before the process runs.129) PEO coupled with electrophoretic deposition (PEO-EPD) is gaining much attention for producing composite coatings with better properties than PEO.130) As a result, the properties of PEO coatings on Al, Mg and Ti substrate can be improved by adding nanoparticles such as HAp, ZrO2, Al2O3, TiO2, and CeO2.131)

Further advances in the PEO treatments for biomedical implants.
Since the PEO coatings always have a porous structure, as follows from its mechanism,132) the corrosion resistance and bioactive properties can be further improved by a pore sealing treatments with an organic compound top coating. Superhydrophobic composite PEO-coatings on titanium and magnesium obtained by application of polytetrafluoroethylene (UPTFE) for the PEO coating pore filling reduces the corrosion rate by 4 orders of magnitude and more; this helps to control inflammatory response of a body.133–135) By using polyethylene glycol methacrylate (PEGMA), the superhydrofobization of coatings was achieved to provide good hemocompatibility.136) A study conducted for the PEO coating on titanium with the functionalization by chitosan showed an improvement of the corrosion properties.137)
Significant progress in improving the corrosion properties of magnesium implants, including nanostructured, has been achieved with the creation of combined coatings based on PEO and biocompatible polymers - polyesters or polysaccharides. Among synthetic biocompatible and bioresorbable polymers, aliphatic polyesters, such as polylactic acid (PLA), poly-lacto-glycolic acid (PLGA), polycaprolactone (PCL), polyethyleneimine (PEI) and many other polymers are of the most interest.138) These polymeric materials provide very attractive coating options for PEO modified coarse-grained and nanostructured Mg and its alloys, since they allow controlling the initial dissolution rate of the biodegradable implant; this depends, among other things, on the nature and molecular weight of the polymer. The plasma electrolytic oxidation of the magnesium surface creates a porous surface that improves the adhesion of polyesters and significantly decreases the corrosion rate.139–142) An in vivo study showed that this method of the surface modification provided sufficient time for bone healing and promotion of new bone growth.143) Improved corrosion resistance of magnesium after its modification by PEO and non-toxic organic additives - hexamethylenetetramine and mannitol was shown as well.144) That makes these coatings promising for biomedical applications on nanostructured Mg implants. Hydrophilic surface was obtained by PEO with a natural polysaccharides hyaluronic acid (HA) and carboxymethylcellulose (CMC) in order to improve bonding function.145) The PEO treatment improves the corrosion resistance by inducing chemical bonding with the biopolymer and sealing the bending of the porous oxide. The combination of PEO with HA exhibited the best retentive ability after implantation. Moreover, the introduction of HA-CMC cross-linked hydrogel into the PEO layer provided the self-healing of localized damage and stable osteocytes in long term bone regeneration.
Therefore, the direction of filling the PEO pores on nanostructured metal implants either with the nanoparticles or the bioactive organic molecules follows the biomimetic approach to the development of advanced coatings, and a significant scientific outcome is expected in this field.
Thus, the results of recent studies presented in this overview convincingly attest to the fact that metallic biomaterials (Ti and Ti alloys, stainless steels and other) after their nanostructuring by severe plastic deformation techniques demonstrate improved mechanical and functional properties, and this accounts for the considerable interest in their medical applications. The latter is also confirmed by the proceedings of the International Workshop on Giant Straining Process for Advanced Materials (GSAM2018) held in 2018 at Kyushu University (JAPAN) and devoted to the significance of severe plastic deformation for the production of biomedical and biocompatible materials.146)
Improved mechanical properties of nanomaterials create solid opportunities for manufacturing of medical implants with improved design and miniaturized devices. The examples of first successful applications of dental and maxillofacial nanoimplants made of nanostructured titanium are considered and discussed in the overview. The paper also highlights the works on the development of continuous SPD processing, in particular, ECAP-Conform technique, designed for mass production of nanotitanium and other nanometals.
The study of biomedical properties of SPD-processed nanometals is a relatively new task because nanostructuring introduces certain advanced features in the materials biological response. At the same time, the results of recent studies on surface modification, including chemical etching and deposition of bioactive coatings, make it a promising approach for the development of advanced medical implants and devices with improved design and biofunctionality.
The authors (RZV) gratefully acknowledge the financial support from Russian Science Foundation (project No. 19-49-02003), and through the RFBR project 17-03-01042 (EVP). We would like to sincerely thank all our colleagues cited in the list of references and, in particular, Prof. Terry Lowe for numerous joint studies on the subject.