2022 Volume 63 Issue 8 Pages 1144-1150
2022 Volume 63 Issue 8 Pages 1144-1150
Chemical alterations of bentonite are critical factors for the ultra-long-term stabilization of barriers and backfill in radioactive waste disposal. The chemical alterations may deteriorate the properties of bentonite-based materials. However, experimental studies on the effects of chemical alterations on the swelling characteristics and permeability of bentonite-based materials have not been investigated extensively. In this study, the effects of chemical factors on the swelling properties and permeability of bentonite-based materials were clarified based on one-dimensional swelling-pressure, hydraulic conductivity, X-ray powder diffraction patterns, and element mapping images. From the analytical and experimental results, a small amount of K+, Fe3+, and Mg2+ can significantly deteriorate the swelling characteristic and permeability of bentonite-based materials in geological disposal (i.e., cause a decrease in swelling-pressure and an increase in hydraulic conductivity). Therefore, we conclude that it is necessary to pay close attention to the existence of these cations.
This Paper was Originally Published in Japanese in J. Soc. Mater. Sci., Japan 71 (2022) 395–401.
Bentonite is thought to have been formed millions to hundreds of millions of years ago by volcaniclastic sediments subjected to physicochemical and hydrothermal alterations on a geological scale because of increased temperatures and pressures.1) Bentonite is a clay material whose main component is montmorillonite, a type of smectite, which is accompanied by silicate, carbonate, sulfate, and sulfide minerals. Bentonite is said to be a “clay material with 1,000 uses” because of its characteristics, such as swelling, thickening, dispersibility, and adsorptivity,2) and its use is widespread in the fields of civil engineering, agriculture, and the chemical industry. In particular, because of these characteristics, the use of sodium-type bentonite as a component of artificial barrier materials for the geological disposal of radioactive waste has been studied in recent years. Thus far, numerous studies on the swelling, water permeability, and self-sealing properties, among others, of bentonite-based materials have been conducted.
In the geological disposal of radioactive waste, the effect of alteration on artificial barrier materials is an important consideration in achieving ultra-long-term safety for thousands to millions of years.3,4) It has been reported that some of the montmorillonite in bentonite may be transformed into illite and chlorite, whereas feldspar may be transformed into kaolinite by undergoing alteration.4,5) For this reason, many studies have evaluated the physical properties of bentonite that has undergone mineralogical changes. In addition, to predict the long-term effects of chemical alteration on bentonite-based materials, a number of numerical simulations6–8) have been performed, whereas experimental studies on the effects of Ca(OH)2 solution and Fe2+ on the water permeability of bentonite9–11) have been conducted. Because cement-based materials, such as grout, are used during construction, and because of concerns about the corrosion of overpacks, it is important to consider the effects of calcium hydroxide and ferrous ions (Fe2+) on bentonite-based materials.
It has been established that groundwater at a depth of at least 300 m, which is the target depth of geological disposal, has no dissolved oxygen, and that the corrosive environment is a non-oxidizing reducing atmosphere. However, unless the underground cavity is backfilled, steel supports and bedrock used in a geological disposal structure will be exposed to an oxidizing environment for a long period of time. Therefore, it is important to pay attention to the ferric ions (Fe3+) retained underground. There has been a study12) on examining the effect of overpack corrosion on the adsorption and diffusion of radionuclides in bentonite and the occurrence of ferric iron corrosion products. However, only a few studies have examined the effects of ferric ions on the swelling characteristics and permeability of bentonite-based materials.
If a variety of aqueous solutions generated from geological disposal sites are eluted because of the influence of various groundwaters, the bentonite-based materials in this environment will naturally be chemically affected, and their physical properties may deteriorate (e.g., decrease in swelling-pressure, increased hydraulic conductivity, and deterioration of self-sealing performance). In particular, it has been reported that the montmorillonite content in bentonite is strongly influenced by the mass transfer of the buffer material (i.e., hydraulic conductivity and effective diffusivity).13,14) In previous studies, the degree of chemical alteration of bentonite materials was often expressed based on the difference in the concentration of the solution used.15,16) However, to the best of our knowledge, there have been no experimental studies on the swelling characteristics and permeability of bentonite based on variations in the montmorillonite content ratio due to chemical alteration.
In this study, we first investigated the effects of chemical factors (in particular, differences in solution type) on the swelling characteristics and permeability of sodium-type bentonite that was immersed in various solutions to promote alteration reactions (the product of which is altered bentonite). Subsequently, when part of the montmorillonite in bentonite (herein, the bentonite-based material is a mixed material composed of sodium-type bentonite and silica sand) was altered by these reactions, which typically occur over the course of ultra-long-term use, the effect of the degree of alteration on the permeability was investigated. Specifically, a constant-pressure permeability test was conducted using specimens in which the altered bentonite was replaced in various proportions, with the assumption that the montmorillonite content would decrease because of the alteration of part of the montmorillonite in the bentonite. Based on the obtained experimental results, the effect of the substitution rate of the altered bentonite on the permeability of bentonite-based materials was determined using the results of powder X-ray diffraction analysis and element mapping analysis.
The sodium-type bentonite used in this study was from Tsukinuno, Yamagata, Japan (Kunigel-V1 by Kunimine Industries Co., Ltd.; particle density is 2.715 Mg/m3). Kunigel-V1 is composed of montmorillonite (46 to 49%), quartz (29 to 38%), feldspars (2.7 to 5.5%), calcite (2.1 to 2.6%), dolomite (2.0 to 2.8%), zeolites (3.0 to 3.5%), and pyrite (0.5 to 0.7%).17) The altered bentonite was prepared via the immersion and stirring of 50 g of sodium-type bentonite (dried at 40°C for 24 h using a constant-temperature dryer from AS ONE Corporation’s EO-700B; natural convection type) in 4 types of 1 L solutions, which will be described later. A variety of solutions were prepared through the addition of solutes to solvent to produce concentrations of 0.1 mol/L; herein, it is important to note that the molar concentrations differed depending on the solubility of the solute. After stirring, the sample was dried at 40°C for 24 h and then made into a powder of size 75 µm or less using an electric mill. For this study, we selected four types of solutions to simulate geological disposal: Ca(OH)2 solution (pH = 12.8) and KOH solution (pH = 13.0), based on the assumption that highly alkaline pore water is generated through the reaction of cement-based material with groundwater; FeCl2 solution (pH = 4.9), based on the assumption that ferric iron (Fe3+) is generated by the reaction between oxygen, which is carried from the ground by excavations performed in facility construction, and iron, which is in the steel support and bedrock; and MgCl2 solution (pH 7.5), based on the assumption that metal ions are present in seawater groundwater in coastal areas. The reason for using an FeCl2 solution despite an assumption based on ferric iron (Fe3+) is as follows: According to a previous study18) that examined the effect of iron hydroxide in a groundwater scenario in geological disposal, iron ions dissolved in groundwater as ferrous iron may be oxidized to form ferric iron after moving through cracks in the bedrock. Furthermore, the solubility of iron hydroxide (Fe(OH)3) in water is extremely low. Therefore, in this study, the sample was stirred in a FeCl2 solution and then dried to obtain ferric iron. The colors of the sodium-type bentonite powder immersed in these solutions changed to milky white in the Ca(OH)2 solution, pale cream in the KOH solution, brown in the FeCl2 solution, and grayish-brown in the MgCl2 solution. Moreover, the bentonite immersed in the FeCl2 solution was browned by drying, which confirmed that the ferrous ions were oxidized into ferric ions. Figure 1 shows the powder X-ray diffraction (XRD) pattern (scanning range: 5° to 10°), focusing on the 2θ = 7.1° (d = 12.4 Å) diffraction peak of the montmorillonite in the sodium-type bentonite and the altered bentonite. XRD was performed using a Rigaku Ultima IV diffractometer (40 kV, 20 mA) with Cu-Kα radiation, 0.15 mm receiving slit, 0.5° divergence slit, and 2° scattering slit. In these patterns, the 12.4 Å diffraction peak of montmorillonite of bentonite immersed in the solution included samples with smaller peaks, samples with diminished peaks, and samples with peak positions that shifted to the lower-angle side.

Unoriented X-ray powder diffraction (XRD) patterns of sodium-type bentonite and bentonite immersed in various solutions.
The sample ratios of the bentonite-based materials were 70% for the sodium-type bentonite and altered bentonite and 30% for the silica sand (Mikawa No. 6 silica sand; particle density is 2.663 Mg/m3). Moreover, the replacement ratio α of the mixed bentonite immersed in the solutions was defined by the ratio of sodium-type bentonite to altered bentonite (Fig. 2). In this study, constant-pressure permeability tests were performed at ratios α = 0%, 25%, 50%, 75%, and 100%.

Proportions of components in bentonite-sand mixtures. α is mixed bentonite immersed in various solutions replacement ratio to bentonite.
For both tests, each specimen was prepared via static compaction of a sample that had been oven-dried at 40°C into a cylinder with a diameter of 50 mm and height of 10 mm, resulting in a density of 1.4 Mg/m3. Both tests were performed in a constant-temperature bath made of heat-insulating material in a constant-temperature room (22 ± 1°C) using the same equipment used in previous studies.3,19) Past research3,19) has confirmed that there were no large variations in the experimental values obtained using either apparatus for the same specimen. Therefore, in this study, only one experiment was performed under the same conditions.
The one-dimensional swelling-pressure test was performed on single samples of altered bentonite. In the swelling-pressure test, each specimen was immersed in distilled water with the volume change constrained. Afterward, the load F generated in the vertical direction was measured at 1-s intervals for 7 days, and the swelling-pressure Ps (= F/A, where A is the specimen cross-sectional area) was calculated. On the other hand, a constant-pressure permeability test was performed on single samples of altered bentonite and bentonite-based materials (α = 0–100%). Each specimen was degassed for two days using a water-immersed decompression vessel and soaked in water for two days under atmospheric pressure, after which the constant-pressure permeability test was performed. With regard to the single samples of altered bentonite, the specimen used in the permeability test was the same as that subjected to the one-dimensional swelling-pressure test. In the permeability test, distilled water was passed through the specimen at a constant water pressure using an air compressor and a pressure water tank. The hydraulic conductivity k (= QL/hAt, where Q is the runoff volume, L is the height of the specimen, h is the difference in water level, A is the cross-sectional area of the specimen, and t is the measurement time) was calculated based on the assumption that Darcy’s law holds. The runoff volume Q was measured by a computer at intervals of 60 s using an analytical balance and was measured for 24 h or more after the first confirmation of the runoff volume. The difference in the water level h was calculated based on the pressure inside the pressure water tank. Please refer to Kohno et al.3) and Kohno19) for details on the test equipment and test method.
Figure 3 shows the results of the one-dimensional swelling-pressure tests on sodium-type bentonite and altered bentonite. The swelling-pressure of sodium-type bentonite was based on data from three experiments (average value of 0.48 MPa) conducted by Kohno et al.3) Excluding the Ca(OH)2- and MgCl2-solution-immersed bentonite, it can be observed that the equilibrium swelling-pressure was reached in approximately 2 days after the start of the test.

Swelling-pressure of sodium-type bentonite and bentonite immersed in various solutions.
For all specimens, the swelling-pressure of bentonite immersed in the solution was lower than that of sodium-type bentonite. The swelling-pressure of the Ca(OH)2-solution-immersed bentonite was 0.26 MPa, which was approximately half that of the sodium-type bentonite. On the other hand, the swelling-pressures of bentonite immersed in the other solutions were approximately 0.03 to 0.04 MPa, which were significantly lower than that of sodium-type bentonite. Therefore, the magnitude of the swelling-pressure of the altered bentonite differed depending on the type of solution where it was immersed. The generation of swelling-pressure in sodium-type bentonite is considered to be caused by increases in the distance between unit layers due to the absorption of large amounts of water between the layers of montmorillonite during water immersion. This is well documented in many references.1,2) On the other hand, as will be described later, the decrease in the swelling-pressure of bentonite immersed in the solution is considered to be due to the exchange of cations between the layers of montmorillonite. In particular, the swelling-pressures Ps of the KOH-, FeCl2-, and MgCl2-solution-immersed bentonites, which exhibited significant reductions in swelling-pressure, were similar to the swelling-pressures of non-swelling clay minerals (mica clay mineral: Ps = 0.02 MPa, talc: Ps = 0.01 MPa, chlorite: Ps < 0.002 MPa)3) under the same test conditions. Therefore, the swelling-pressures of bentonite immersed in the KOH, FeCl2, and MgCl2 solutions were not due to the absorption of water between the layers of montmorillonite. Instead, the swelling-pressures of bentonite immersed in the KOH, FeCl2, and MgCl2 solutions can be considered to have been caused mainly by increases in pore water pressure during water immersion, similar to in non-swelling clay minerals.
Figure 4 shows the relationship between swelling-pressure and hydraulic conductivity. According to the figure, the larger the swelling-pressure, the smaller the hydraulic conductivity. The hydraulic conductivity of sodium-type bentonite is based on data from three experiments conducted by Kohno et al.3) In this study, sodium-type bentonite exhibited the smallest hydraulic conductivity (k = 5.3 × 10−13 m/s). This is approximately the same as the hydraulic conductivity (k = 4.8 × 10−13 m/s) obtained in previous studies20) under the same test conditions. On the other hand, although the pH values of both the Ca(OH)2- and KOH-solution-immersed bentonites were similar, there was a large difference in the swelling-pressure or hydraulic conductivity between them; therefore, it can be inferred that the dissolved ion species have an effect on these properties. Bentonite immersed in the different solutions exhibited higher hydraulic conductivities than that of sodium-type bentonite. The hydraulic conductivities of bentonite immersed in KOH, FeCl2, and MgCl2 solutions (k = 4.7 × 10−9 m/s, k = 4.0 × 10−9 m/s, and k = 1.1 × 10−8 m/s, respectively) were approximately 104 times higher than that of sodium-type bentonite. However, by comparison, the hydraulic conductivity exhibited by the bentonite immersed in Ca(OH)2 solution (k = 4.2 × 10−12 m/s) was only approximately 10 times that of bentonite. One of the reasons for this is that Na+ between the layers of montmorillonite in the sodium-type bentonite was exchanged with cations in the different solutions. The cations (Na+) between the layers are not strongly electrically bonded and are easily exchanged for other cations. Ion selectivity (exchange invasion input) is greater for Ca2+ than for Na+.21) In the Ca(OH)2-solution-immersed bentonite, the exchange of Na+ with Ca2+ is inferred to be characterized by restricted intercalation, resulting in a decrease in swelling-pressure and an increase in hydraulic conductivity. This can be confirmed from the X-ray diffraction pattern (Fig. 1), which shows that the 12.4 Å diffraction line of montmorillonite is slightly smaller in the Ca(OH)2-solution-immersed bentonite. By contrast, the following can be confirmed for the bentonite immersed in the other solutions: The 12.4 Å diffraction line of montmorillonite was extinguished in the bentonite immersed in the KOH solution, whereas in the FeCl2- and MgCl2-aqueous-solution-immersed bentonite, the 12.4 Å diffraction line of montmorillonite moved to the low-angle side (Fig. 1). For this reason, in the bentonite immersed in the KOH, FeCl2, and MgCl2 solutions, the montmorillonite was dissolved, and the crystal structure was disturbed or destroyed by immersion. Consequently, the swelling-pressure decreased significantly, the voids in the sample could not be filled sufficiently, and the hydraulic conductivity increased significantly.

Relationship between swelling-pressure and hydraulic conductivity of sodium-type bentonite and bentonite immersed in various solutions.
These experimental results suggest that the swelling characteristics and permeability of bentonite-based materials used in geological disposal deteriorate because of the effects of alteration, and that the degree of deterioration varies depending on the quality of the groundwater in contact.
3.2 Effect of replacement ratio of altered bentonite on permeability of bentonite-based materialsFigure 5 shows the relationship between the replacement ratio of altered bentonite and hydraulic conductivity. The hydraulic conductivity3) of the bentonite-based material (α = 0%; mixed material composed of sodium-type bentonite and silica sand) is 1.4 × 10−12 m/s. This is approximately the same as the hydraulic conductivity (1.0 × 10−12 m/s)20) reported in previous studies under the same test conditions. In addition, the hydraulic conductivity of the bentonite-substituted sample immersed in the Ca(OH)2 solution at α = 100% is only approximately 10 times higher than that of the bentonite-based material (α = 0%). By contrast, the hydraulic conductivities of the bentonite-substituted samples immersed in the KOH, FeCl2, and MgCl2 solutions at α = 100% were significantly higher (approximately 104 to 105 times) than that of the bentonite-based material (α = 0%). Although the hydraulic conductivities of single samples of bentonite immersed in these solutions, as shown in the previous section, increased because of the mixing of silica sand, the magnitude relationship between them was the same. In all specimens, the hydraulic conductivity tended to increase as the replacement ratio of the altered bentonite increased; however, the paths of the graphs differed depending on the type of altered bentonite to be substituted. Of particular note is that although the hydraulic conductivities of the FeCl2- or MgCl2-solution-immersed bentonite and of the KOH-solution-immersed bentonite were similar (α = 100%), there was a large difference in hydraulic conductivity (α = 25% and α = 50%) between the former and the latter. In the FeCl2- and MgCl2-solution-immersed bentonite substitution samples, the hydraulic conductivities were approximately 103 times higher than that of bentonite-based materials (α = 0%), even with a small substitution rate of α = 25%. Therefore, in this study, the reason behind the differences in hydraulic conductivity of these samples was determined using element mapping images.
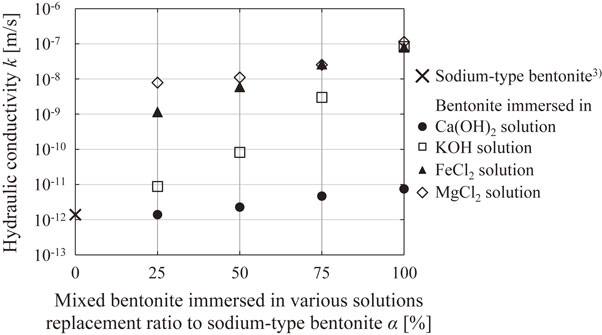
Relationship between mixed bentonite immersed in various solutions replacement ratio to sodium-type bentonite and hydraulic conductivity.
Element mapping is a qualitative analysis method that can visually confirm the elemental components and their distribution states using characteristic X-rays emitted when a sample is irradiated with an electron beam.22) In this study, element mapping was performed using a field-emission scanning electron microscope (Hitachi High-Technologies Corporation, S-4800, Energy Dispersive X-ray Spectroscopy SEM-EDX, Detector: OXFORD X-Max). The proportions of the component elements were then determined using the obtained images. The degree of influence of chemical factors on sodium-type bentonite, specifically with regard to substitution with altered bentonite, was investigated via element mapping analysis, and the relationship between these results and the permeability of the sample was investigated.
As shown in Fig. 6, the specimen was a two-layered cylinder with a diameter of 50 mm and height of 10 mm. The upper part of the specimen was a bentonite (altered) layer with a thickness of 3 mm, which was immersed in one of the different solutions, whereas the lower part of the specimen was a sodium-type bentonite (unaltered) layer with a thickness of 7 mm. Similar to the samples used in the one-dimensional swelling-pressure test and the constant-pressure permeability test specimen, the specimens for this test were statically compacted to a density of 1.4 Mg/m3. The right arrows in Fig. 6 and Fig. 7 indicate the boundaries between the altered and unaltered bentonite. The prepared specimen was soaked in water for 7 days in the same manner as in the one-dimensional swelling-pressure test, and element mapping analysis of the cross sections of the specimen was performed. Subsequently, the effect of altered bentonite on sodium-type bentonite in the unaltered part was investigated.

Cross-section photo of two-layer-integrated bentonite specimen and their element (Ca, K, Fe, Mg) mapping. Upper layers of A to D photo are bentonite immersed in Ca(OH)2, KOH, FeCl2 and MgCl2 solutions.

Percentage of elements in the element (Ca, K, Fe, Mg) mapping.
Figures 6(A) to (D) show a range of 1-cm squares of the cross section cut in the diameter direction of the specimen after water immersion. In particular, in the specimen in which the upper layer was composed of FeCl2-solution-immersed bentonite, it can be visually observed that the sodium-type bentonite portion of the lower layer was brownish and greatly affected by ferric ions. On the other hand, with regard to the sodium-type bentonite part in the lower layers of the other specimens, the occurrence of discoloration, among others, cannot be observed with the naked eye. The element mapping images of corresponding 2-mm squares at the altered/unaltered boundary of these sample cross sections are shown at the bottoms of Figs. 6(A)–(D). Furthermore, the 2-mm square element mapping images were analyzed at 0.1-mm intervals in the z-direction. Figure 7 shows a plot of the proportions of elements present in each region (length 0.1 mm × width 2 mm). The broken line in Fig. 7 shows the proportions of the elements present in the unaltered part before water immersion. According to the figure, each of the cation elements (Ca, K, Fe, Mg) from the different solutions was detected in the altered upper layer of each sample. In the specimen with bentonite that was immersed in the Ca(OH)2 solution, the boundary between the altered and unaltered layers can be clearly confirmed (Fig. 6). In the unaltered part, the amount of Ca was approximately equal to the initial value before water immersion. Thus, in the Ca(OH)2-solution-immersed bentonite-substituted sample, the mobility of Ca to the unaltered part due to mixing with the altered bentonite was not as large as those of K, Fe, and Mg. This is considered to be the reason behind the relatively low increase in hydraulic conductivity of the Ca(OH)2-solution-immersed bentonite. On the other hand, in the specimens with bentonite that was immersed in the KOH, FeCl2, or MgCl2 solution, the boundary between the altered and unaltered layers was unclear (Fig. 6). These elements were also detected in the unaltered layer, and there were no significant differences in the proportions of these elements detected before and after the altered/unaltered boundary. Therefore, compared to Ca, these elements were inferred to be characterized by greater mobilities toward bentonite in the unaltered layer, even when mixed in small amounts. This is considered to be one of the factors that significantly increased the permeability coefficient of sodium-type bentonite. However, in the specimen with bentonite that was immersed in the KOH solution, the proportion of K gradually decreased in the z-direction from the boundary. At z = 2 mm, the ratio was almost the same as the initial value before immersion. From this, it was inferred that the mobility of the element to the unaltered part was smaller than those of the specimens with bentonite that was immersed in either the FeCl2 or MgCl2 solution. This may be one of the factors that caused the large difference in the hydraulic conductivity of the KOH-solution-immersed bentonite (α = 25% and α = 50%) relative to the hydraulic conductivities of the FeCl2- and MgCl2-solution-immersed bentonite-substituted samples. In the future, it will be necessary to conduct a permeability test of the two-layer specimens used in element mapping analysis.
Subsequently, the degree of influence of the altered bentonite on the unaltered portion due to differences in element mobility/adsorption, ion valence, and solution concentration at the boundary between the altered bentonite and Na-type bentonite was investigated. From this, the details of the relationship between the swelling characteristics and permeability of the mixed specimen can be clarified.
When bentonite-based materials are used for geological disposal, the elution of Ca2+ is probably remarkable because of the influence of cement-based materials, such as grout. From our experimental results, it was inferred that K+ (which is present in highly alkaline pore water), Fe3+ (metal ions generated by the reaction between oxygen carried from the ground and iron in the support and bedrock), and Mg2+ (metal ions present in seawater groundwater) can be among the factors that greatly deteriorate the performance of bentonite materials, even when these ions occur in small amounts. Therefore, in geological disposal applications, it will be necessary to pay close attention to the presence of these cations (K+, Fe3+, and Mg2+).
In this study, a one-dimensional swelling-pressure test and a constant-pressure permeability test were conducted to clarify the swelling characteristics and permeability of the following specimens: bentonite (altered bentonite) specimens that have been immersed in various solutions to promote alteration reactions, and specimens in which the altered bentonite was replaced in various proportions with respect to the bentonite-based materials. The results are summarized as follows.
This work was partly supported by Grants-in-Aid for Scientific Research ‘KAKENHI’ (grant number 19K15489) from the Japanese Society for the Promotion of Sciences (JSPS). We gratefully acknowledge this support. The authors thank Mr. Hiroki Uchida and Mr. Naoto Senkoji from Tottori University for their assistance with the experiments and analysis. The authors also thank the two reviewers for their helpful comments and suggestions.