2017 Volume 93 Issue 8 Pages 648-655
2017 Volume 93 Issue 8 Pages 648-655
A mixture of (E,Z)-isomers of methyl 12-trishomofarnesoate (methyl 3,7,11-trimethyl-2,6,10-pentadecatrienoate), a juvenile hormone mimic, was synthesized in nine steps (32.6% overall yield) by starting from only four commercially available materials: 2-hexanone, vinylmagnesium bromide, methyl acetoacetate and trimethyl phosphonoacetate. The mimic is useful in increasing the yield of silk by elongating the larval period of the silkworm, Bombyx mori (L.).
Juvenile hormones (JHs) are important in insect life and have a dual function: in juvenile forms as status quo hormones, and in adults they show a number of regulatory tactics.2)–4) Fifty years ago in 1967, Röller isolated JH I (Fig. 1) from Cecropia moth and its structure was established by Trost by the synthesis of its racemate.5) Later studies revealed JHs to be a group of epoxy esters generated by homologation and epoxidation of methyl farnesoate. Preparation of JHs was a prosperous area of organic synthesis, and culminated in the enantioselective synthesis of JH I and others.1),6),7)

Structures of juvenile hormone I and related compounds.
Synthesis of JH mimics was also studied extensively, hoping that some of them might be utilized as insect growth regulators (IGRs) to control the pest insects, because JH mimics prolong the larval stage and inhibit normal metamorphosis to afford reproductive adults.2)–4)
In Japan we developed quite a different application of JH and JH mimics in connection with the silkworm, Bombyx mori (L.). Since ancient days sericulture was important in Eastern Asia around China. Silk was highly estimated as “Queen of the Textiles”.8) In 1872, only five years after the Meiji revolution, the huge brick-made buildings of Tomioka Silk Mill were constructed by the Japanese government under the leadership of a Frenchman named Brunat, and the excellent Japanese silk was exported to all over the world. The buildings were well preserved until now, and in 2014 the mill was inscribed on the World Heritage List by UNESCO (http://www.tomioka-silk.jp).
In the case of Bombyx mori (L.), the naturally occurring (10R,11S)-(+)-JH I was highly bioactive while its opposite enantiomer was inactive,9) Recent LC-MS analysis proved JH I to be the major JH in hemolymph of the silkworm.10) Aside from such basic studies, application of JH in sericulture was attempted by several groups, because prolongation of the larval stage by JH might result in the formation of larger fifth instar larvae and hence the production of larger cocoons. The increase of silk protein would enable the harvest of more than normal amount of silk filaments.
In 1971 Akai and Kiguchi showed that Mori’s synthetic JH I and 13-homo JH I, both as (E,Z)-mixtures (see Fig. 1), increased the accumulation of silk protein by Bombyx mori (L.).11) Then in 1972, by using the same two compounds, Nihmura et al. carried out a large-scale sericultural experiment to show definitely the utility of JHs in increasing the silk production (about 30% increase).12) Nihmura’s method was successfully tested by Ajinomoto/Katakura joint venture in a pilot plant. Unfortunately, however, the Japanese government did not give permission to widely use the new JH technology coupled with artificial diet containing only a minimal amount of mulberry leaves. The method was considered to be harmful to the benefit of 250,000 sericultural farms in Japan in 1975, because it would revolutionize the traditional sericulture. It should be added that Tamura and his co-workers also observed the increase of silk production by administration of their own JH mimic (Fig. 1).13) JH studies related to sericulture were reviewed by Akai et al. in 1973.14)
Due to the socioeconomic change and rural depopulation, the number of Japanese sericultural farms decreased rapidly. Japan’s production of silk cocoons was 400,000 tons in 1930s as harvested by 2,200,000 sericultural farms. It was only 135 tons in 2015 with about 300 farms. The present situation is critical to our kimono and silk textile industries. The time has come to revive the JH technology in sericulture.
The present paper reports in detail a simple and scalable new synthesis of methyl 12-trishomofarnesoate (1, Fig. 1. IUPAC name: methyl 3,7,11-trimethyl-2,6,10-pentadecatrienoate) as the JH mimic practically useful in sericulture.
Refractive indices were measured on an Atago DMT-1 refractometer. IR spectra were measured on a Jasco FT/IR-410 spectrometer. 1H NMR spectra (400 MHz, TMS at δ = 0.00 as the internal standard) and 13C NMR spectra (100 MHz, CDCl3 at δ = 77.0 as the internal standard) were recorded on a Jeol JNM-ECZ 400S/L1 spectrometer. GC-MS were measured on Agilent Technologies 5975 inert XL. HRMS were recorded on Jeol JMS-T100GCV. Column chromatography was carried out on Merck Kieselgel 60 Art 1.00734. All of the 1H and 13C NMR data reported in the present work refer to those obtained with mixtures of (E,Z)-isomers.
2.2. 3-Methyl-1-hepten-3-ol (5).A solution of 2-hexanone (4, 20.0 g, 200 mmol) in dry THF (20 mL) was added dropwise to a stirred and ice-cooled solution of vinylmagnesium bromide (2) in THF (1 M, 220 mL, 220 mmol) at 5–20 °C. The mixture was stirred at 0–5 °C for 15 min and at 40 °C for 40 min. The reaction was then quenched by the addition of ice and NH4Cl solution, and the mixture was extracted with Et2O. The Et2O extract was washed successively with water and brine, dried (MgSO4), and concentrated under atmospheric pressure through a Vigreux column. The residue was distilled to give 5 (22.0 g, 86%) as a colorless oil, bp 83–87 °C/6.4 kPa; nD24 = 1.4346; νmax (film): 3395 (br. m), 3087 (w), 2959 (s), 2934 (s), 2873 (m), 1644 (w), 1467 (m), 1412 (m), 1371 (m), 1159 (m), 1128 (m), 996 (m), 920 (s) cm−1; δH(CDCl3): 0.90 (3H, t, J 6.8 Hz), 1.28 (3H, s), 1.28–1.36 (4H, m), 1.47 (1H, br. s), 1.49–1.58 (2H, m), 5.04 (1H, dd, J 1.2, 10.8 Hz), 5.19 (1H, dd, J 0.8, 17.2 Hz), 5.91 (1H, dd, J 10.8, 17.2 Hz); GC-MS [column: HP-5 ms, 5% phenylmethylsiloxane, 0.25 mm × 30 m; carrier gas, He; press 61 kPa; temp: 70–230 °C (+10 °C/min)]: tR 3.44 min (99.5%); MS (70 eV, EI): m/z: 128 (<1) [M+], 113 (8), 101 (5), 85 (4), 71 (100), 55 (8), 43 (22). HRMS calcd for C8H16O: 128.1201, found: 128.1206.
2.3. 3-Methyl-2-heptenyl bromide (6).The neat alcohol 5 (22.0 g, 172 mmol) was added dropwise to an ice-cooled and vigorously stirred 47% HBr (120 mL) at 5–10 °C. After stirring for 1 h at 0–5 °C, the mixture was extracted with pentane. The pentane extract was washed successively with water, NaHCO3 solution and brine, dried (MgSO4), and concentrated in vacuo. The residue was distilled to give 29.6 g (90%) of 6 as a colorless oil, bp 108–112 °C/6.4 kPa; nD24 = 1.4838; νmax (film); 2958 (s), 2931 (s), 2871 (m), 2861 (m), 1656 (m), 1455 (m), 1379 (m), 1200 (s), 849 (m), 587 (m) cm−1; δH(CDCl3): 0.90 and 0.93 (total 3H, both t, J 7.2 Hz), 1.24–1.50 (4H, m), 1.72 and 1.76 (total 3H, both s), 2.05 and 2.13 (total 2H, both t, J 7.2 Hz), 3.99–4.12 (2H, m), 5.25 (1H, t-like, J 8 Hz); GC-MS (same conditions as used for 5) tR 6.60 (33.9%), 6.86 min (66.0%); MS of (Z)-6 with tR = 6.60 min (70 eV, EI): m/z: 192 (2), 190 (2), 111 (92), 95 (5), 81 (11), 79 (9), 69 (100), 55 (82), 41 (38); MS of (E)-6 with tR = 6.86 min (70 eV, EI): m/z: 190 (2), 188 (2), 111 (100), 95 (4), 81 (10), 79 (9), 69 (97), 55 (77), 41 (35). HRMS of 6 with a longer tR calcd for C8H15Br: 190.0357, found: 190.0355.
2.4. 6-Methyl-5-decen-2-one (8).The bromide 6 (29.5 g, 155 mmol) was added to a stirred mixture of methyl acetoacetate (34.8 g, 300 mmol) and K2CO3 (60.0 g, 435 mmol) in acetone (150 mL) and DMF (40 mL). The reaction was exothermic, and the temperature of the mixture raised to ca. 40 °C. When the exothermic reaction ceased, the mixture was stirred and heated under reflux for 1 h. It was then concentrated in vacuo. The residue was diluted with water, and extracted with Et2O. The Et2O solution was washed successively with water and brine, dried (MgSO4), and concentrated in vacuo to give 39.3 g of a crude oil containing 7, νmax (film); 2956 (s), 2931 (s), 2872 (m), 2860 (m), 1747 (s), 1718 (s), 1651 (w), 1629 (w), 1437 (m), 1360 (m), 1245 (m), 1201 (m), 1150 (s), 1040 (w) cm−1. The oil was then dissolved in MeOH (100 mL), and a solution of NaOH (14.0 g, 350 mmol) in water (100 mL) was added to it. The mixture was stirred and heated under reflux for 1 h. It was then concentrated in vacuo. The residue was diluted with water, and extracted with Et2O. The Et2O extract was washed successively with water and brine, dried (MgSO4), and concentrated in vacuo. The residue was distilled to give 20.9 g (81%) of 8 as a colorless oil, bp 71–77 °C/0.2 kPa, nD23 = 1.4474; νmax (film); 2958 (s), 2929 (s), 2872 (m), 2860 (m), 1718 (s), 1624 (w), 1456 (m), 1359 (m), 1158 (m) cm−1; δH(CDCl3): 0.89 and 0.90 (total 3H, both t, J 7.2 Hz), 1.20–1.42 (4H, m), 1.60 and 1.66 (total 3H, both s), 1.95 and 2.02 (total 2H, both t, J 7.6 Hz), 2.14 (3H, s), 2.22–2.34 (2H, m), 2.40–2.52 (2H, m), 5.06 (1H, t-like, J 6.8 Hz); GC-MS (same conditions as those used for 5) tR 8.57 (35.4%), 8.79 min (64.6%); MS of (Z)-8 with tR = 8.57 min (70 eV, EI): m/z: 168 (19) [M+], 150 (22), 111 (37), 110 (68), 95 (55), 81 (62), 69 (45), 68 (51), 55 (44), 43 (100); MS of (E)-8 with tR = 8.79 min (70 eV, EI): m/z: 168 (20) [M+], 150 (24), 111 (42), 110 (73), 95 (56), 81 (59), 69 (47), 55 (44), 43 (100). HRMS of 8 with a longer tR calcd for C11H20O: 168.1514, found: 168.1514.
2.5. 3,7-Dimethyl-1,6-undecadien-3-ol (9).A solution of the ketone 8 (15.1 g, 90 mmol) in dry THF (20 mL) was added dropwise over 10 min to a stirred and ice-cooled solution of vinylmagnesium bromide (1 M in THF, 100 mL, 100 mmol) at 10–20 °C. The mixture was stirred for 15 min at 5–10 °C and then stirred and heated at 40 °C for 40 min. It was then cooled, treated with ice and NH4Cl solution, and extracted with Et2O. The Et2O solution was washed successively with water and brine, dried (MgSO4), and concentrated in vacuo. The residue was distilled to give 15.6 g (89%) of 9 as a colorless oil, bp 91–98 °C/0.2 kPa; nD24 = 1.4624; νmax (film); 3389 (br. m), 3086 (w), 2960 (s), 2928 (s), 2859 (m), 1640 (w), 1457 (m), 1376 (m), 1107 (m), 995 (m), 920 (s) cm−1; δH(CDCl3): 0.88 and 0.90 (total 3H, both t, J 6.8 Hz), 1.21–1.40 (4H, m), 1.28 (3H, s), 1.50–1.66 (3H, m), 1.58 (2H, s), 1.66 (1H, s), 1.92–2.10 (4H, m), 5.06 (1H, d, J 10 Hz), 5.09–5.16 (1H, m), 5.21 (1H, d, J 17 Hz), 5.91 (1H, dd, J 10, 17 Hz); GC-MS (same conditions as those used for 5): tR 9.93 (34.5%), 10.32 min (65.5%); MS of 9 with tR = 9.93 min (70 eV, EI): m/z: 178 (10) [(M-H2O)+], 135 (5), 121 (39), 107 (14), 93 (100), 81 (31), 80 (39), 79 (22), 71 (64), 69 (47), 55 (48), 43 (38), 41 (32). MS of 9 with tR = 10.32 min was almost identical with the above data. HRMS of 9 with a longer tR calcd for C13H24O: 196.1827, found: 196.1828.
2.6. 3,7-Dimethyl-2,6-undecadienyl bromide (10).The neat alcohol 9 (15.5 g, 79 mmol) was added dropwise to a vigorously stirred and ice-cooled 47% HBr (95 mL) at 5–10 °C. After stirring for 1 h at 0–5 °C, the mixture was extracted with hexane. The hexane extract was washed successively with water, NaHCO3 solution and brine, dried (MgSO4), and concentrated in vacuo. The residual 10 (23.1 g, quant.) was a colorless oil, nD24 = 1.4922; νmax (film); 2957 (s), 2929 (s), 2860 (m), 1656 (w), 1595 (w), 1455 (m), 1378 (m), 1200 (m), 990 (w), 894 (m), 846 (w) cm−1; δH(CDCl3): 0.90 (2H, t, J 7.2 Hz), 0.91 (1H, t, J 7.2 Hz), 1.58 (3H, s), 1.67 (3H, s), 4.01 (2H, d, J 8 Hz), 4.90–5.65 (2H, m). GC-MS (same conditions as those used for 5): tR 8.46 (4.4%), 8.75 (4.8%), 12.76 (8.9%), 13.04 (35.6%), 13.26 min (41.6%). MS of 10 with tR = 13.26 min (70 eV, EI): m/z: 258 (<1), 179 (14), 111 (46), 93 (25), 81 (23), 79 (13), 69 (100), 55 (51), 41 (22). Other bromides showed similar MS. Distillation caused decomposition of 10, giving trienes. The bromide 10 should be used without distillation. HRMS of 10 with the longest tR calcd for C13H23Br: 258.0983, found: 258.0981.
2.7. 6,10-Dimethyl-5,9-tetradecadien-2-one (12).The bromide 10 (23.0 g, prepared from 79 mmol of 7) was added to a stirred mixture of methyl acetoacetate (17.4 g, 150 mmol) and K2CO3 (29.0 g, 210 mmol) in acetone (100 mL) and DMF (20 mL). The reaction was exothermic. When the exothermic reaction ceased, the mixture was stirred for 40 min at room temperature, and then for 1 h under reflux. It was then concentrated in vacuo. The residue was diluted with water, and extracted with Et2O. The Et2O solution was washed successively with water and brine, dried (MgSO4), and concentrated in vacuo to give 29.5 g of a crude oil containing 11, νmax (film): 2956 (s), 2930 (s), 2860 (m), 1748 (s), 1719 (s), 1632 (w), 1437 (m), 1244 (m), 1151 (m) cm−1. The oil was then dissolved in MeOH (75 mL), and a solution of NaOH (8.8 g, 220 mmol) in water (75 mL) was added to it. The mixture was stirred and heated under reflux for 1 h. It was then concentrated in vacuo. The residue was diluted with water, and extracted with Et2O. The Et2O extract was washed successively with water and brine, dried (MgSO4), and concentrated in vacuo. The residue was distilled to give 14.9 g (80%, 2 steps) of 12 as a colorless oil, bp 115–130 °C/0.2 kPa; nD24 = 1.4672; νmax (film); 2958 (s), 2929 (s), 2859 (m), 1719 (s), 1455 (m), 1359 (m), 1158 (m) cm−1; δH(CDCl3): 0.88 (2H, t, J 6.8 Hz), 0.90 (1H, t, J 6.8 Hz), 1.20–1.50 (4H, m), 1.60, 1.65 and 1.66 (total 6H, each s), 1.90–2.10 (6H, m), 2.12 (3H, s), 2.20–2.30 (2H, m), 2.40–2.50 (2H, m), 5.08 (2H, br); GC-MS (same conditions as those used for 5): tR 14.01 (7.6%), 14.28 (36.6%), 14.52 min (41.6%). MS of 12 with tR = 14.01 min (70 eV EI): m/z: 236 (1) [M+], 151 (19), 110 (37), 95 (79), 81 (61), 69 (84), 68 (28), 67 (22), 55 (68), 43 (100), 41 (34). MS of 12 with tR = 14.28 min (70 eV, EI): m/z: 236 (1) [M+], 151 (21), 125 (13), 107 (14), 95 (15), 93 (13), 69 (100), 67 (13), 55 (60), 43 (70), 41 (21). MS of 12 with tR = 14.52 min (70 eV, EI): m/z: 236 (1) [M+], 178 (11), 151 (28), 125 (20), 107 (23), 93 (13), 69 (100), 67 (14), 55 (59), 43 (83), 41 (21). HRMS of 12 with the longest tR calcd for C16H28O: 236.2140, found: 236.2134.
2.8. Methyl 3,7,11-trimethyl-2,6,10-pentadecatrienoate (1).A solution of trimethyl phosphonoacetate (13, 14.6 g, 80 mmol) in dry THF (10 mL) was slowly added dropwise to a stirred suspension of NaH (60% NaH, 3.2 g, 80 mmol) in dry THF (50 mL) and dry DMF (60 mL) at room temperature with occasional cooling with an ice-bath. When the evolution of H2 ceased, a solution of the ketone 12 (14.8 g, 63 mmol) in dry THF (20 mL) was added dropwise to the stirred mixture, which was stirred for 2 d at room temperature. The mixture was diluted with ice and water (300 mL), and extracted with Et2O. The Et2O extract was washed successively with water and brine, dried (MgSO4), and concentrated in vacuo. The residue (21.2 g) was chromatographed over SiO2 (200 g). Elution with hexane/EtOAc (10:1) gave 16.1 g of crude 1, which was distilled to afford 13.4 g (73%) of 1 as an (E/Z)-isomeric mixture (colorless oil), bp 145–155 °C/0.2 kPa; nD25 = 1.4800; νmax (film); 2955 (s), 2929 (s), 2859 (m), 1722 (vs), 1651 (m), 1435 (m), 1224 (m), 1148 (s), 857 (w) cm−1; δH(CDCl3): 0.88 and 0.90 (total 3H, both t, J 7.2 Hz), 1.20–1.50 (4H, m), 1.57–1.80 (6H, m), 1.88–2.20 (8H, m), 2.16 (5H, s), 3.67 (3H, s), 5.05–5.20 (2H, m), 5.65 (1H); δC(CDCl3): 4.09, 15.94, 18.90, 22.38, 23.44, 26.02, 26.61, 30.27, 39.43, 41.02, 50.83, 115.25, 115.83, 122.92, 123.91, 135.43, 135.74, 135.91, 136.24, 166.25, 166.79, 167.34; GC-MS (same conditions as those used for 5): tR 16.91 (2.5%), 17.12 (5.5%), 17.26 (7.3%), 17.35 (5.7%), 17.47 (12.6%), 17.58 (14.6%), 17.63 (16.3%), 17.88 min (35.4%). The isomer with tR = 17.88 min seems to be (2E,6E,10E)-1. MS of 1 with tR = 17.88 min (70 eV, EI): m/z: 292 (3) [M+], 261 (3), 235 (3), 207 (5), 178 (5), 121 (18), 114 (51), 111 (22), 110 (21), 95 (17), 81 (36), 69 (100), 67 (16), 55 (56), 41 (19). HRMS of 1 with the longest tR calcd for C19H32O2: 292.2402, found: 292.2393.
2.9. Administration of 1 on Bombyx mori (L.).The experiments were done at Kimono Brain Co., Ltd. (Tokamachi, Niigata, Japan) employing the procedures recorded in ref. 12. The result shown in Fig. 5 was obtained by rearing 3,145 larvae of Bombyx mori (L.).
Since 1969 we carried out SAR (structure-activity relationship) studies on JH mimics A (Fig. 2).15)–24) We found A with R1=n-Bu, R2=R3=R4=Me to be of high JH activity against allatectomized fourth instar larvae of Bombyx mori (L.).20),22) JH mimics B with an aromatic ring were also synthesized and evaluated.24) The B-type mimics, however, were larvicidal, even without the epoxy ring as in the case of the mimic C (Fig. 2).25),26) Accordingly, the ester-type mimics must be more suitable.
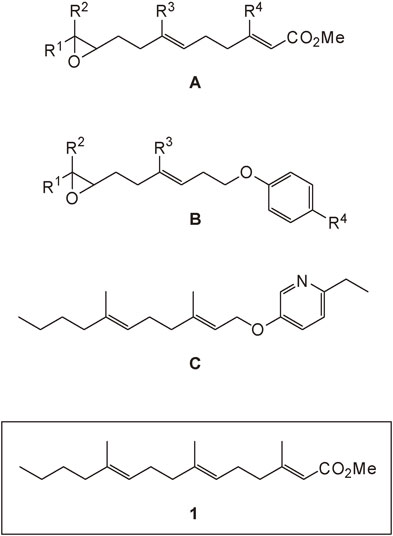
Compound 1 was selected as the practical JH mimic useful in sericulture. Compounds of A and B types are unstable due to the presence of the epoxy group. Compounds of B and C types are larvicidal. These compounds were all prepared and bioassayed as stereoisomeric mixtures.
The presence of the epoxy ring in A makes the mimic less stable against moisture owing to its possible hydrolysis giving the corresponding diol. Artificial diet containing a JH mimic is made by heating the foodstuff at 90 °C in the presence of water.12) It is therefore undesirable to use JH mimics A in large-scale sericulture. Fortunately, JH mimic 1 is known to be about 100 times more active than JH I.23) The final decision was therefore made to use a stereoisomeric mixture of 1 for sericulture.
3.2. Choice of the most practical synthetic route to JH mimic 1.The JH mimic 1 was prepared twice in the past. In 1973 the cyclopropyl alcohol D (Fig. 3) was subjected to Julia cleavage to give bromide E, which eventually yielded 1.20) In 1975 organocopper chemistry was employed to convert F to 1.22) The problems in these past syntheses were the use of commercially unavailable cyclopropane-containing building block and that of the unstable organocopper reagent.

Synthetic strategies leading to 1. The strategy adopted in the present work gives a simple and scalable solution.
In the present work more practical and simpler strategy as shown in the sequence G → H → I → J is adopted, although it seems rather classical and conventional. Accordingly, iterative use of commercially available vinylmagnesium bromide (2), 47% hydrobromic acid, and methyl acetoacetate (3) enables us to prepare 1 from all commercially available 2-hexanone (4, Fig. 4), 2, 3 and trimethyl phosphonoacetate (13, Fig. 4) in only nine steps as detailed below.

Synthesis of a stereoisomeric mixture of methyl 12-trishomofarnesoate (1).

Oral administration of the JH mimic 1 remarkably increases the size of the silkworm cocoons at the dosage of 0.6 µg/larva, when given to the fifth instar larvae. Left: Control (no addition of 1); Right: Addition of 1 (courtesy of Mr. T. Kobayashi, Kimono Brain Co., Ltd., Tokamachi-shi, Niigata).
Figure 4 summarizes the new synthesis of methyl 12-trishomofarnesoate (1, methyl 3,7,11-trimethyl-2,6,10-pentadecatrienoate) as a stereoisomeric mixture.
Addition of vinylmagnesium bromide (2) to 4 gave 5, which was treated with 47% hydrobromic acid to furnish allylic bromide 6. Alkylation of methyl acetoacetate (3) with 6 in the presence of potassium carbonate in acetone and DMF27) afforded 7, whose alkaline hydrolysis and decarboxylation gave unsaturated ketone 8 as an (E,Z)-mixture. Then the same sequence of vinylation/hydrobromic acid treatment/acetoacetic ester synthesis was repeated to give 12 via 9, 10 and 11. Although the preparation of 10 was reported previously by us,26) the conditions for each of the synthetic steps were further optimized in the present work. The ketone 12 was obtained as a mixture of the four (E,Z)-isomers. Previous GC-analytical works indicated that compounds with (E)-double bond(s) show longer retention times (tRs) than their (Z)-isomers.5),15),22) Accordingly, the major isomer (41.6%) of 12 must be (6E,10E)-12, because it showed the longest tR (14.28 min).
Finally, Horner-Wadsworth-Emmons olefination of 12 with trimethyl phosphonoacetate (13) furnished 1 as a mixture of the eight possible (E,Z)-isomers. The major isomer (35.4%) with the longest tR (17.88 min) was most probably (3E,7E,11E)-1. The overall yield of 1 based on 4 was 32.6% (nine steps). Within five working days over 10 g of 1 could be prepared routinely. Repetition of the synthesis guaranteed the reproducibility of the above process without any trouble.
3.4. Effect of JH mimic 1 on the size of cocoons produced by silkworms reared with artificial diet containing 1.As shown in Fig. 5, 0.6 µg/larva of 1 mixed in the artificial diet12) prolonged the larval period of the fifth instar larvae of Bombyx mori (L.) for three days, and increased the weight of the cocoon layer (30–40% increase). Details of the biological experiments will be published separately. At present it is unclear whether 1 is bioactive as it is or shows bioactivity after biological epoxidation at the double bond at C-10. Only after clarifying the X-ray structure of the JH/receptor complex, we will be able to discuss the details of SAR of JH and JH mimics. We have to wait until we know more about the JH receptor of insects.28)
A new, efficient and easy-to-execute synthesis of an (E,Z)-mixture of methyl 12-trishomofarnesoate (1) was achieved by starting from four commercially available materials: 2-hexanone (4), vinylmagnesium bromide (2), methyl acetoacetate (3) and trimethyl phosphonoacetate (13). The JH mimic 1 was used practically for sericulture at Kimono Brain Co., Ltd., Tokamachi-shi, Niigata, to increase the size of silkworm cocoons.
I thank Mr. M. Kimura (President, Toyo Gosei Co., Ltd.) and Mr. Y. Kimura (CEO, Toyo Gosei Co., Ltd.) for their support. I am indebted to Mr. M. Okamoto (President, Kimono Brain Co., Ltd.) for his suggestion to undertake the present work. Mr. Y. Shikichi and Dr. K. Sakaguchi (both Toyo Gosei Co., Ltd.) are thanked for NMR and GC-MS measurements. Dr. T. Nakamura (RIKEN) kindly carried out the HRMS analysis. Dr. T. Tashiro (Niigata University of Pharmacy and Applied Life Sciences) and Mr. K. Kikusato (RIKEN) helped me in preparing the manuscript. My thanks are due to Mr. T. Kobayashi (Kimono Brain Co., Ltd.), Mr. S. Aomori (ex-Katakura Kogyo Co.), and Dr. M. Nihmura (ex-Ajinomoto Co., Inc.) for their laborious works in silkworm rearing.