2018 Volume 246 Issue 1 Pages 59-64
2018 Volume 246 Issue 1 Pages 59-64
Distal symmetric polyneuropathy, represented by chronic inflammatory demyelinating polyneuropathy, is a popular neurological condition. Some cases are known to be associated with genetic mutations or serum auto-antibodies, but the exact mechanisms in most cases are unknown. Recently, osmotic factors have been suggested to trigger some neurological disorders, such as neuromyelitis optica. The aim of the present study was to assess the possible association of osmotic factors in the pathogenesis of distal polyneuropathy. We prospectively measured the serum levels of osmolality, electrolytes, total protein, albumin, blood urea nitrogen, glucose, and osmolality gap in the patients with acute distal polyneuropathy before treatments (n = 12) and those with other comprehensive neurological disorders such as multiple sclerosis and neurodegenerative diseases (n = 176). Then, we compared each osmotic fraction between the two groups. As a result, all of the 12 patients with acute distal polyneuropathy, including 4 patients with chronic inflammatory demyelinating polyneuropathy, showed abnormally high or low values of osmolality gap, compared to the others (p < 0.0001, F-test). In the patients with other diseases, there were 2 patients with abnormally high osmolality gap values, which were attributable to their hyperlipidemia or high titer of serum autoantibody unrelated to polyneuropathy. In conclusion, serum osmolality gap would be elevated or decreased in the acute phase of distal symmetric polyneuropathy. Osmotic imbalance between the serum and nerve cells, based on abnormal excess or deficit of some unidentified serum osmolytes, may be one of the mechanisms in symmetric polyneuropathy with unknown causes.
Distal symmetric polyneuropathy is a popular neurological condition presenting dysesthesia and/or paresis with distal predominance. Some part of the patients have known causes like genetic mutations or neuropathy-related serum autoantibodies, but most of the patients do not have known causes (Lehmann et al. 2009; Saporta and Shy 2013). At present, the diagnosis of polyneuropathy is largely dependent on nerve conduction study, but the abnormal findings in nerve conduction study are not specific to neurological diseases in the peripheral nervous system (Franssen and van den Bergh 2006). As a result, proper diagnosis and therapeutic intervention in the early phase of polyneuropathy is sometimes quite difficult (Misra et al. 2008).
In some neurological diseases in the central nervous system (CNS), serum osmotic change has been known to be one of the triggers of their onsets (Martin 2004; King and Rosner 2010). Recently, osmotic imbalance between serum and CNS tissue has been suggested to cause damages to glia cells (Akaishi et al. 2018a, b). Also in the peripheral nerves, several case reports showed that dehydration, hemodialysis, and pregnancy, all of which affects serum osmolality, could trigger distal symmetric polyneuropathy (Tegner and Lindholm 1985; Nardin et al. 2005; Hojs-Fabjan and Hojs 2006). However, until now, there has been no study evaluating the associations between osmotic factors and distal symmetric polyneuropathy with unknown causes. In this study, we prospectively studied the possible associations between osmotic factors and distal symmetric polyneuropathy, trying to elucidate the mechanism in the distal polyneuropathy without known causes.
Serum osmolality, together with the serum levels of sodium, blood urea nitrogen (BUN), and glucose, were measured in the inpatients on planned admission at Tohoku University Hospital who required comprehensive laboratory data screening and agreed to participate in this study between November 2015 and March 2017. Data were acquired from each patient only once. All serum samples were collected before the intravenous treatments on admission and all laboratory data were measured in the department of clinical laboratory at Tohoku University Hospital.
Enrolled patients and diagnosis of neuropathyA total of 188 consecutive inpatients with neurological symptoms agreed to participate in this study and their serum osmolality fractions were comprehensively measured. All the enrolled patients were with good appetite and were well-nourished under oral intake. The average age of the enrolled patients was 51.6 ± 17.6 years old; 85 were male and 103 were female.
Among the enrolled 188 patients, 12 patients were eventually diagnosed with distal symmetric polyneuropathy, including chronic inflammatory demyelinating polyneuropathy (CIDP), presenting distal-dominant neurological symptoms symmetrically in the four extremities. The diagnoses of polyneuropathy in all the patients with suspected neuropathy were confirmed by the findings in nerve-conduction study (NCS) that matched their clinical manifestations.
Measurement of serum osmolality gapSerum osmolality was measured in all enrolled patients by the method of freezing-point depression. This method is based on the fact that freezing-point depression of a solution (Δt) [K] is proportionate to the molality (m) [mol/kg] of the resolved compounds.
Δt = Kf m
Here, Kf is the freezing point depression constant for each type of the used solvent [K kg/mol]. For example, Kf for water (H2O) is about 1.86 [K kg/mol]. With this formula, we estimated the molality of solutes from the measured Δt.
Serum sodium concentration was measured with LaboSPECT 008 (Hitachi High-Technologies Corporation, Tokyo, Japan), utilizing the sodium-ion selective electrode method. Serum osmolality was measured with OM-6050 OsmoStation (ARKRAY, Kyoto, Japan). The measuring equipment was calibrated every morning using one solution for standardization. Osmolality gap (OG) and theoretical values of the calculated osmolality were estimated by the equations described below (Dorwart and Chalmers 1975; Brownlow and Hutchins 1982; Purssell et al. 2001). Serum concentration of sodium was measured in [mEq/l] and those of BUN and glucose were measured in [mg/dl].
OG [mOsm/kg] = (measured serum osmolality) – (calculated osmolality)
 |
Comparison of the studied variables between the patients with polyneuropathy and the others was performed with Student’s t-test for the averages and F-test for the standard deviations. Because multiple variables were simultaneously compared, p-values less than 0.01 were regarded as statistically significant. Statistical analyses in this study were conducted using SPSS Statistics Base 22 software (IBM, Armonk, NY, USA).
Ethics committee approvalThe protocol of this study and all the procedures used were approved by the Institutional Review Board of Tohoku University Hospital. Written informed consents were acquired from all the enrolled patients.
Among the enrolled 188 patients, 12 patients were finally diagnosed with distal symmetric polyneuropathy in the acute phase, 5 patients were with Charcot-Marie-Tooth disease, 2 patients were with mononeuropathy multiplex related to systemic vasculitis, and the other 169 patients were with other diagnoses of miscellaneous neurological disorders, such as multiple sclerosis (n = 28), Parkinson disease or multiple system atrophy (n = 14), amyotrophic lateral sclerosis (n = 12), neuromyelitis optica spectrum disorders (n = 12), psychosomatic disorder (n = 8), epilepsy (n = 4), and so on. The clinical features and the results of nerve conduction study in the 12 patients with distal polyneuropathy with unknown causes are summarized in Table 1. Among the 12 patients with distal polyneuropathy, 4 patients were diagnosed with chronic inflammatory demyelinating polyneuropathy (CIDP) with elevated protein levels in the cerebrospinal fluid.

Details of the 12 patients with acute distal polyneuropathy with unknown causes.
All of the patients with peripheral neuropathy who showed abnormal serum osmolality gap (OG) were diagnosed with distal polyneuropathy without certain cause like genetic mutations. On the other hands, all of the patients with Charcot-Marie-Tooth disease or mononeuropathy multiplex (not shown in this table) showed normal serum OG.
A, axonal disturbance; CIDP, chronic inflammatory demyelinating polyneuropathy; D, demyelination; F, female; IgM, immunoglobulin M; M, male; MMN, multifocal motor neuropathy; n.a., not available; OG, osmolality gap [mOsm/kg]; PGB, pregabalin; Tx, treatment.
Serum osmotic factors, including osmolality, OG, sodium ion, albumin, BUN, and glucose were compared between the patients with acute distal polyneuropathy with unknown causes (n = 12) and the others (n = 176). In this study, the group of 176 patients with neurological diseases other than distal polyneuropathy is tentatively called as “the control group” for convenience.
Among the studied osmotic factors, only the distribution of OG was significantly different between the two groups (Fig. 1A). The distribution of OG in distal polyneuropathy was deviated to abnormally high or low range, compared to the distribution in the control group (p < 0.0001, F-test). Based on the distribution of OG in the control group, the suggested normal range of OG, within which 95% of population will fit, would be roughly between 0.50 and 7.80. For reference, all of the 12 patients with acute distal polyneuropathy were located outside this normal range. Among the 176 patients of the control group, two patients showed abnormally high OG values, as shown in Fig. 1A. The diagnosis of the patient with OG of 9.63 was encephalitis related to serum anti-glutamic acid decarboxylase antibody with the titer > 300,000 U/ml. The diagnosis of the other patient with OG of 9.43 was untreated severe hyperlipidemia with the serum triglyceride of 346 mg/dl.
As shown in Fig. 1B, the distribution of serum sodium ion in polyneuropathy group was lower than that in the control group (p = 0.0012, Student’s t-test), but their distributions were mainly overlapped, and serum sodium ion could not explain the abnormally high or low OG values in polyneuropathy. As shown in Fig. 1C, the distribution of serum osmolality in polyneuropathy group was not significantly different from that in the control group (p = 0.0119, Student’s t-test).
As summarized in Table 2, mean ± standard deviation of the other factors (i.e. serum potassium ion, serum chloride ion, BUN, serum creatinine, estimated glomerular filtration rate, total protein, albumin, glucose, hemoglobin A1c, serum triglyceride, thyroid hormones) were not significantly different between the polyneuropathy group and the control group. Though not shown in this table, the distributions of BUN, albumin, and glucose were also largely overlapped and were not significantly different between the two groups.

Distributions of serum osmolality gap, sodium ion, and osmolality in distal polyneuropathy and in the others.
Osmolality gap was abnormally high or low in all of the 12 patients with acute distal polyneuropathy, but showed normal distribution in the others. The distributions of serum sodium ion and osmolality were largely overlapped between those with polyneuropathy and the others.

Comparison of serum laboratory data between those with polyneuropathy and the others.
Shown values with ranges are mean ± SD of the variables. p values are the results of Student’s t-test or Mann-Whitney’s U test.
Cre, serum creatinine level; eGFR, estimated glomerular filtration rate; free T3, serum free triiodothyronine; free T4, serum free thyroxine; HbA1c, hemoglobin A1c; Osm, serum osmolality; TG, serum triglyceride level; TSH, thyroid stimulating hormone.
We compared the clinical and laboratory data between the patients with acute distal polyneuropathy who showed abnormally high OG (n = 5) and those who showed abnormally low OG (n = 7). There were no statistical differences in any of the age, serum osmolality, sodium ion, BUN, glucose, albumin, and body-mass index (p ≥ 0.10 for all, Mann-Whitney’s U test). Statistical difference was confirmed only in the total protein level (7.46 ± 0.39 vs 6.24 ± 0.70; p = 0.0079).
OG values in distal polyneuropathy after the treatments or in the chronic phaseAmong the 12 patients with acute distal polyneuropathy, there was only one patient who was measured for OG both before the intravenous immunoglobulin G (IVIG) treatment and after the treatment. The patient’s OG, which was 10.38 before the IVIG treatment, was decreased to 6.51 after the treatment.
For reference, there were five patients with CIDP, whose OG were measured in the chronic phase or just after the IVIG treatment. Their OG values were 3.88, 5.43, 5.47, 6.51, and 6.88, in the order of numerical size; none of them showed abnormal OG values.
In this study, we studied the serum OG values together with other osmotic fractions in patients with comprehensive neurological diseases. The results of this study suggested that abnormal serum OG is likely to exist in the acute phase of distal polyneuropathy with unknown origins. Such abnormal OG values were not observed in the chronic phase or after the IVIG treatment. Theoretically speaking, OG represents the amount of serum osmotic substances other than sodium, BUN, and glucose. Usually, OG values are measured in emergency medicine, because it reflects exogenous substances in intoxicated patients (e.g., acute alcohol intoxication, drug abuse) (Dawson and Whyte 2001; Purssell et al. 2001; Fenves et al. 2006; Lynd et al. 2008; George and Shannon 2009). However, none of these exogenous factors were suspected or confirmed in the 12 patients with polyneuropathy. We need to explore factors other than exogenous substances to explain the abnormal OG values in the patients with distal polyneuropathy.
Van’t Hoff’s law of osmotic pressure shows that the osmotic pressure of a solution (Π) [Pa] is proportional both to the concentration of resolved compounds (C) [mol/m3] and temperature (T) [K], which can be calculated as below by using an ideal gas molar constant (R) (Schulz 1969).
Π = C R T
Based on this equation, osmotic pressure from extracellular fluid to intracellular fluid would be mainly regulated by the total molarity of the resolved compounds in each of the fluids, no matter of the molecular sizes.
As shown in Fig. 2, whether a substance can freely pass through intravascular and cellular membranes or not mainly depends on its molecular size and on whether the substance have transporters or channels on the membranes. Based on the results of this study, as the cause of abnormal OG in the patients with polyneuropathy, serum electrolytes, albumin, BUN, and glucose were unlikely. Certain other molecules that cannot freely pass through the cellular membrane may be the cause of abnormal OG, which would result in osmolality gradient across the cellular membrane and osmotic pressure-related stress of the nerve cells. Because the nerve cells are likely to show large surface area per volume, compared to other spherical cells, the nerve cells could be more vulnerable to such osmotic pressure-related stress than other cells.
There are some limitations for this study. First, the number of patients with acute distal polyneuropathy (n = 12) in this study was relatively small. Abnormal OG in patients with acute distal polyneuropathy should be validated by different sample set in the future. Another limitation is that the exact mechanism to connect the abnormal OG with the pathogenesis of polyneuropathy has not been elucidated in this study. Some unknown osmolality-related factors, reflected by abnormal OG values, seem to exist behind the pathogenesis of distal polyneuropathy without known causes. Based on the diagnoses of the 2 patients with abnormal OG values in the control group and the theory in Fig. 2, lipoproteins or proteins including autoantibody would not be the cause of polyneuropathy. Glycoprotein, mucopolysaccharides, or ethanol may be one of the candidate molecules. Further researches are needed to identify the serum substance to produce abnormal OG values in polyneuropathy patients.
In conclusion, abnormal OG exists in the acute phase of distal symmetric polyneuropathy, including CIDP. Excess or deficiency of some osmotic fractions would produce pressure gradient between intracellular- and extracellular-fluids, eventually resulting in osmotic pressure-related damages in the nerve cells.
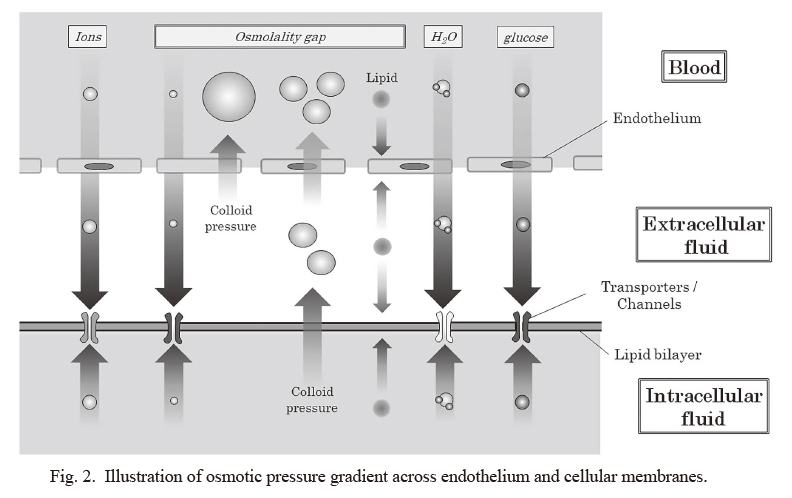
Illustration of osmotic pressure gradient across endothelium and cellular membranes.
This illustration shows the estimated osmolality gradient among blood, extracellular fluid, and intracellular fluid, based on the size and type of molecules. Those substances that can almost freely pass through the endothelium and cellular membrane would not produce osmolality gradient across the surface of nerve cells. Those that cannot freely pass through the membrane would produce osmolality gradient across the cellular surface. Whether an osmotic substance can pass through endothelium and cellular membranes depends on its molecular size and whether it has transporters or channels on them.
The authors thank Mr. Takuya Takeda, Ms. Yuko Abe, and Prof. Mitsuo Kaku (Infection Control and Laboratory Diagnostics, Tohoku University Hospital, Japan) for measuring the serum osmolality of the enrolled patients and also for their professional advices.
The authors declare no conflict of interest.