2015 年 43 巻 4 号 p. 233-237
2015 年 43 巻 4 号 p. 233-237
Using a mouse model, we previously demonstrated that subcutaneous infection with the JaTH160 strain of Japanese encephalitis virus (JEV) causes significantly higher virulence and stronger virus propagation in the brain compared with that of the JaOArS982 strain. We also showed that the JaTH160 strain, but not JaOArS982, expresses the NS1’ protein and that NS1’ enhances JEV production in avian cells and embryonated chicken eggs. In this study, we examined whether NS1’ expression affects virulence in mice infected with the JaOArS982 and JaTH160 strains using the corresponding recombinant viruses S982-IC and JaTH-IC. Expression of the NS1’ protein in S982-IC diminished the mortality in mice, whereas S982-IC viruses without NS1’ caused 40–60% mortality. However, the viral loads in the brains of these mice were not significantly different despite the dvariation in NS1’ expression. JaTH-IC viruses depleted of the NS1’ protein exhibited high mortality levels, similar to those of the virus expressing NS1’. Previous studies showed that the NS1’ protein plays a role in the enhanced virulence of the JEV SA14 strain in mice. However, our current data suggest that NS1’ protein expression in S982-IC reduces, rather than enhances, the mortality in mice. Thus, the effect of NS1’ on pathogenicity in vivo may vary among virus strains. Our data also suggest that the reduced mortality resulting from NS1’ expression in S982-IC is not simply due to viral replication in the brains. Further investigation is needed to uncover the mechanism by which NS1’ affects pathogenicity in JEV-infected animals.
Japanese encephalitis virus (JEV) causes clinical symptoms in humans, including a non-specific febrile illness, meningitis, encephalitis and meningoencephalitis [1, 2]. JEV belongs to the family Flaviviridae, genus Flavivirus [3], which comprises enveloped viruses containing a single-stranded, positive-sense RNA genome of 11 kb in length. The genomic RNA contains a 5′ untranslated region (UTR), a single open reading frame (ORF) and a 3′ UTR. The ORF encodes a long polyprotein, which is co- and post-translationally processed by a combination of viral and cellular proteases into three structural (C, prM and E) and seven nonstructural (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) proteins [4, 5].
To investigate pathologies of the central nervous system (CNS) caused by JEV infection, mouse models have been employed [6, 7]. We previously showed that the virulence of the JaTH160 strain is significantly higher than that of the JaOArS982 strain in mice [7]. Thus, a genetics-based comparison between these strains appears to be an effective approach for identifying viral factors that contribute to disease severity.
Our previous study showed that the JaTH160 strain produces the NS1’ protein (55 kDa) in addition to the NS1 protein (48 kDa), whereas the JaOArS982 strain expresses only NS1 [8]. The NS1’ protein has been detected among members of the JEV subgroup, including West Nile virus (WNV) and Murray Valley encephalitis virus (MVEV) [9–12]. This protein is produced as a result of −1 programmed ribosomal frameshifting (−1PRF) at the conserved slippery heptanucleotide (YCCUUUU) and 3′-adjacent potential pseudoknot near the beginning of the NS2A coding gene [12]. The NS1’ protein co-localizes with the NS1 protein and can substitute for NS1 in WNV replication [13].
We demonstrated that NS1’ protein expression elevates JEV production in avian cells and embryonated chicken eggs [8]. It was shown that abolishing NS1’ reduced WNV production in mosquitos and house sparrows [14]. These findings suggest that the NS1’ protein may facilitate the amplification/maintenance role of birds in the virus transmission cycle in nature.
Using a mouse model, it was shown that the NS1’ protein plays an important role in enhancing the virulence of WNV and JEV [15, 16], although the mechanisms by which NS1’ expression influence viral pathogenicity have not been identified. These findings raise the possibility that NS1’ expression in the JaTH160 strain contributes to its higher virulence in mice compared with the JaOArS982 strain. Here, therefore, we examined whether NS1’ expression affects the difference in virulence between JaOArS982 and JaTH160.
The expression of NS1’ in JEV is regulated by the nucleotides at positions 66 and 67 of the NS2A gene [8, 16]. Thus, we developed NS1’-expressing and -non-expressing viruses, based on the full-length cDNA of the infectious clones S982-IC and JaTH-IC [8], by substitution of nucleotides 66 and/or 67 of the NS2A gene [17] as indicated in Fig. 1. Recombinant viruses of S982_I23-NS1 and JaTH_V23-NS1’ have identical nucleotide sequences of wild type JaOArS982 and JaTH160, respectively. Nucleotide sequence of each virus was confirmed by conventional sequencing. BHK cells were infected with each virus at a multiplicity of infection of 10. The expression of the NS1 and NS1’ proteins was confirmed by western blot analysis, as previously done (Fig. 1) [8]. The anti-NS1 polyclonal antibodies and anti-β-Actin antibodies (Santa Cruz) were used for detection.
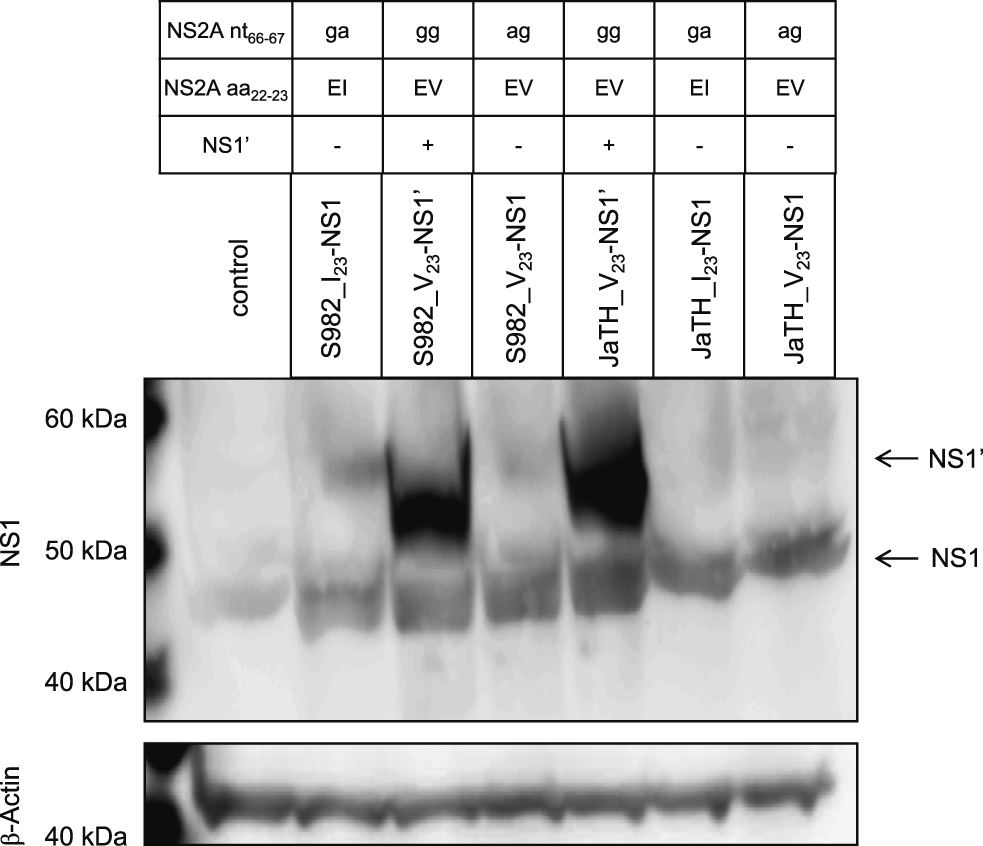
NS1 and NS1’ protein expression. BHK cells were infected with each virus at a multiplicity of infection of 10. Western blotting was performed on the extracted proteins. The anti-NS1 polyclonal antibodies and anti-β-Actin antibodies (Santa Cruz) were used for detection. Mutated nucleotide sequences (NS2A nt66–67) and amino acids (NS2A aa22–23) of each recombinant virus were indicated.
C57BL/6j (B6) mice (Japan SLC Cooperation, Japan CLEA Cooperation) were subcutaneously inoculated with 104 pfu of the recombinant viruses and were observed for clinical signs and lethality. The experimental protocols involving animals were approved by the Animal Care and Use Committee, Nagasaki University (approval number: 091130-2-7/0912080807-9, 100723-1-3/1008050873-3).
We first examined the propagation of recombinant viruses in baby hamster kidney (BHK) and mouse neuroblastoma (N2a) cells. However, there were no significant differences of viral yields in the culture fluids between the viruses (data not shown).
Interestingly, no mice died following infection with S982_V23-NS1’ (Fig. 2A), which caused a slight weight reduction (Fig. 2B). The survival curves and weight changes of the S982_V23-NS1-inoculated mice were similar to those inoculated with S982_I23-NS1 (Fig. 2A and 2B). These observations suggest that NS1’ expression did not increase the mortality in mice infected with JaOArS982 and that NS1’ may in fact reduce virulence. However, there were no significant differences in the viral loads in the brains among the S982_I23-NS1, S982_V23-NS1’ and S982_V23-NS1 viruses at 11 days post-inoculation (pi) (Fig. 2C). These observations suggest that the reduced mortality upon infection with S982_V23-NS1’ was not simply due to viral replication in the brains.
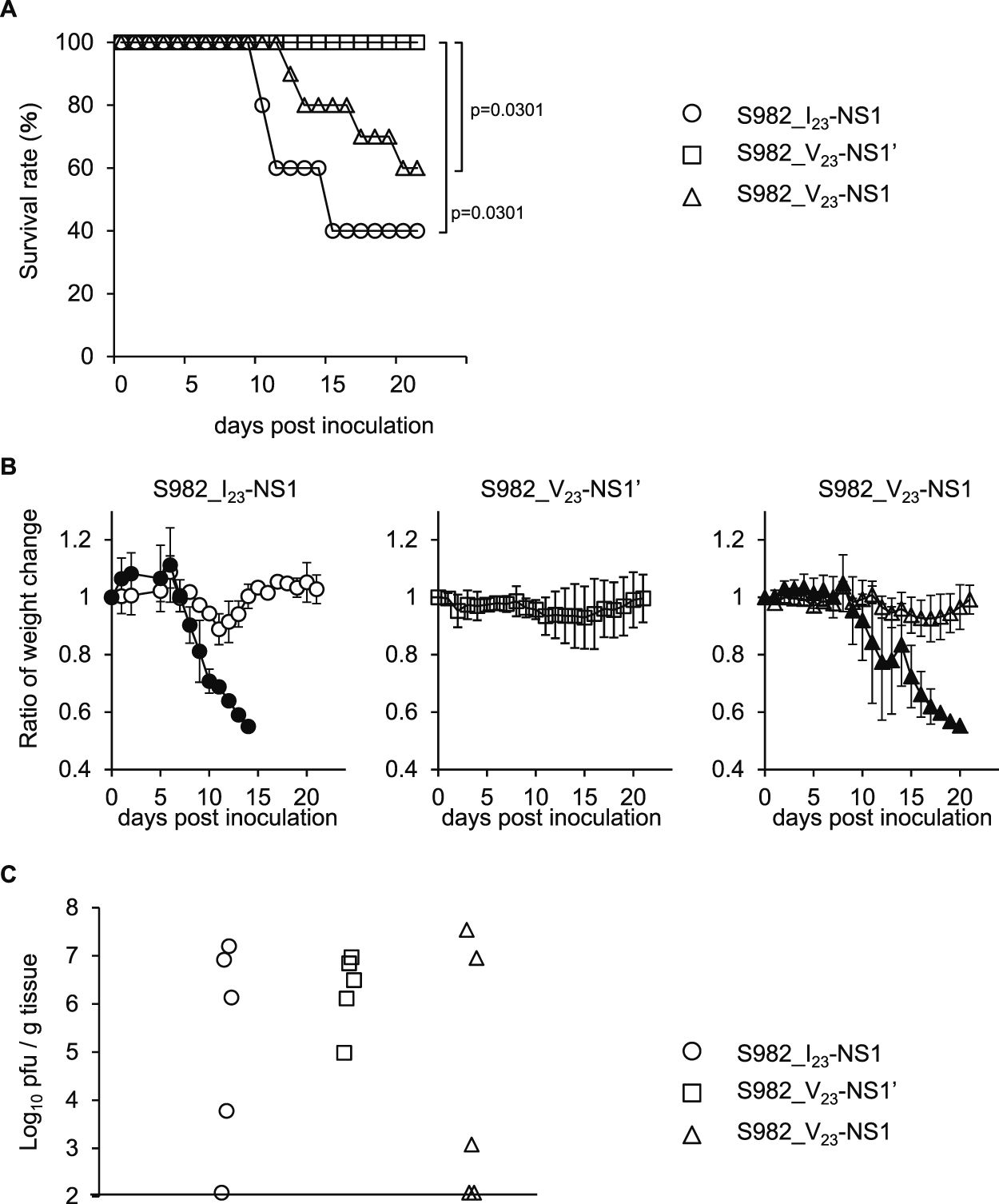
Pathogenicity of S982-IC and derivative viruses in mice. (A) Survival rates of B6 mice following subcutaneous infection with 104 pfu of the S982_I23-NS1 (S982-IC), S982_V23-NS1’ and S982_V23-NS1 viruses. Infected mice were monitored until 21 days post-inoculation (n = 10). p: Gehan-Breslow-Wilcoxon Test. (B) Average weight-change ratios in the groups of live and dead mice at the different time-points compared with those of day 0. Error bars represent the standard deviations. Closed and open symbols identify mice that died or survived, respectively, during the observation period. (C) Viral loads in the brain cortex at 11 days post-inoculation (n = 5).
Following infection with the JaTH_V23-NS1’, JaTH_I23-NS1 and JaTH_V23-NS1 viruses, mouse fatalities were 100%, 90% and 80%, respectively (Fig. 3A). The weight changes of the dead mice were similar among these viruses (Fig. 3B). There were no significant differences in the viral loads in the brains among the mice infected with JaTH_V23-NS1’, JaTH_I23-NS1 and JaTH_V23-NS1 (Fig. 3C). These observations suggest that NS1’ expression did not significantly affect the pathogenicity or viral titers in the CNS in mice infected with the JaTH160 strain.

Pathogenicity of JaTH-IC and derivative viruses in mice. (A) Survival rates of B6 mice following subcutaneous infections with 104 pfu of the JaTH_V23-NS1’ (JaTH-IC), JaTH_I23-NS1 and JaTH_V23-NS1 viruses. Infected mice were monitored until 21 days post-inoculation (n = 10). (B) Average weight-change ratios in the groups of live and dead mice at different time-points compared with those of day 0. Error bars represent the standard deviations. Closed and open symbols identify mice that died or survived, respectively, during the observation period. (C) Viral loads in the brain cortex at 9 days post-inoculation (n = 5).
It has been shown that abolishing the expression of PRF and NS1’ attenuates the virulence of the SA14 strain of JEV in mice [16]. Although the mechanism by which NS1’ affects virulence is still unclear, the functions of NS1’ in pathogenicity may vary among virus strains.
It was recently shown that C-terminal truncation of the NS1’ protein and loss of heat-stable NS1’ dimers of WNV had no effect on viral pathogenesis in mice, suggesting that mammalian hosts are unlikely to be the primary driver for the evolution of the PRF mechanism for NS1’ expression [18]. On the other hand, NS1’-expressing JEV and WNV showed efficient virus production in avian and Culex mosquito hosts, and an important role for PRF in avian and mosquito hosts was demonstrated [8, 14]. Thus, it is suggested that the PRF mechanism is more likely to evolve in avian and mosquito hosts.
Our current study suggests that NS1’ protein expression does not affect neuronal viral infections in mice infected with the JaOArS982 or JaTH160 strains. Further detailed analysis of the different steps in the pathway should help elucidate the precise pathogenic mechanisms of JEV infection.
The authors declare that they have no competing interests.
YT and DH designed the study, YT, MR and DH performed experiments, YT, KM and DH analyzed the data, and YT and DH wrote the paper.
We thank Dr. Tomohiko Takasaki from the National Institute of Infectious Diseases, Tokyo, Japan, for providing the JaTH160 strain. This work was supported by KAKENHI [Grant-in-Aid for Scientific Research (B) (25304045) and Grant-in-Aid for Challenging Exploratory Research (25660229 and 23658243)] from the Japan Society for the Promotion of Science; the Japan Society for the Promotion of Science; the Health and Labor Sciences Research Grant on Emerging and Re-emerging Infectious Diseases from the Japanese Ministry of Health, Labor and Welfare; Research on International Cooperation in Medical Science (Japan-US Cooperative Program), Health and Labor Sciences Research Grants; the Cooperative Research Grant(s) of NEKKEN 2014; AMED and the Japan Initiative for Global Research Network on Infectious Diseases.