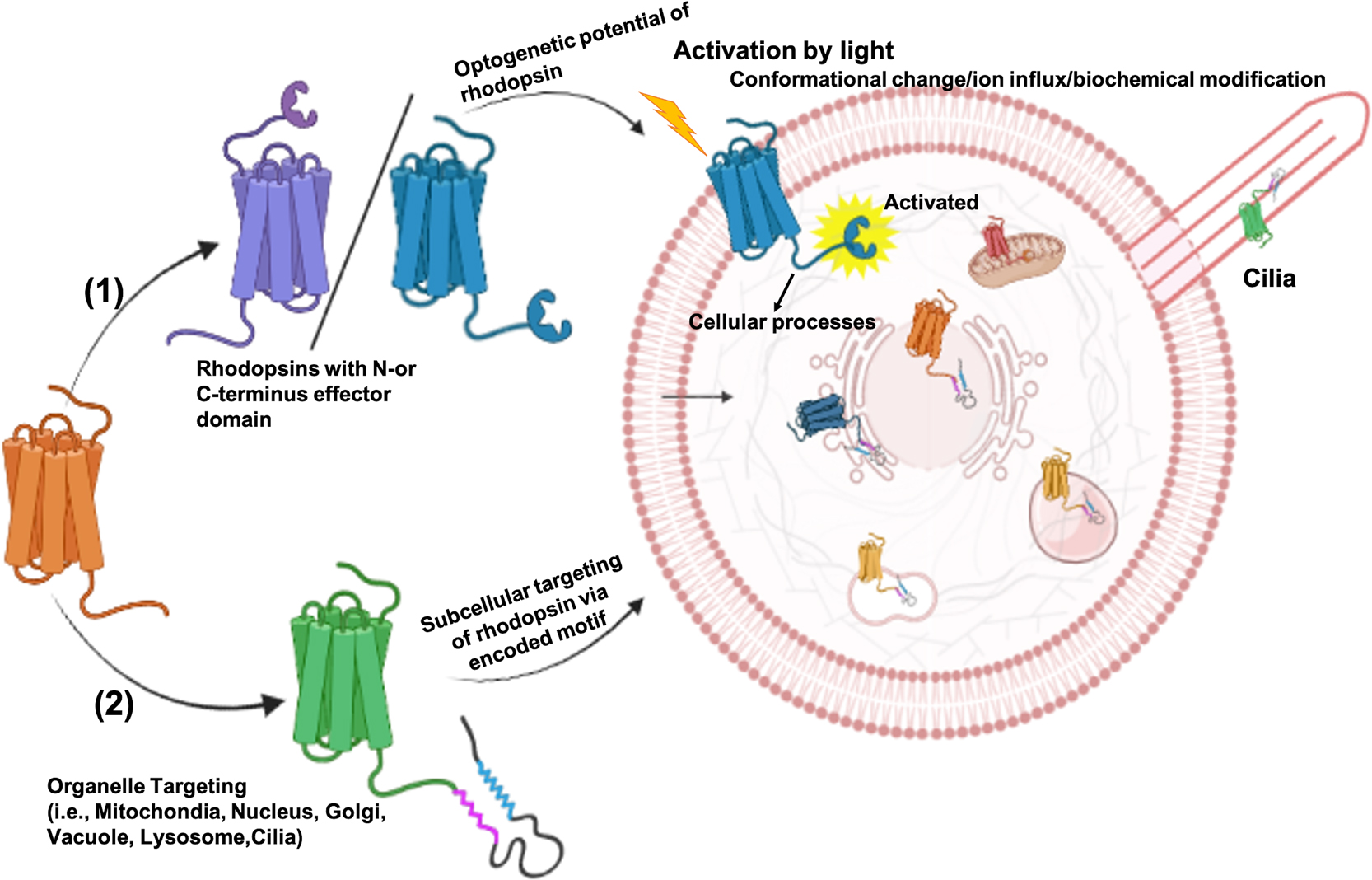
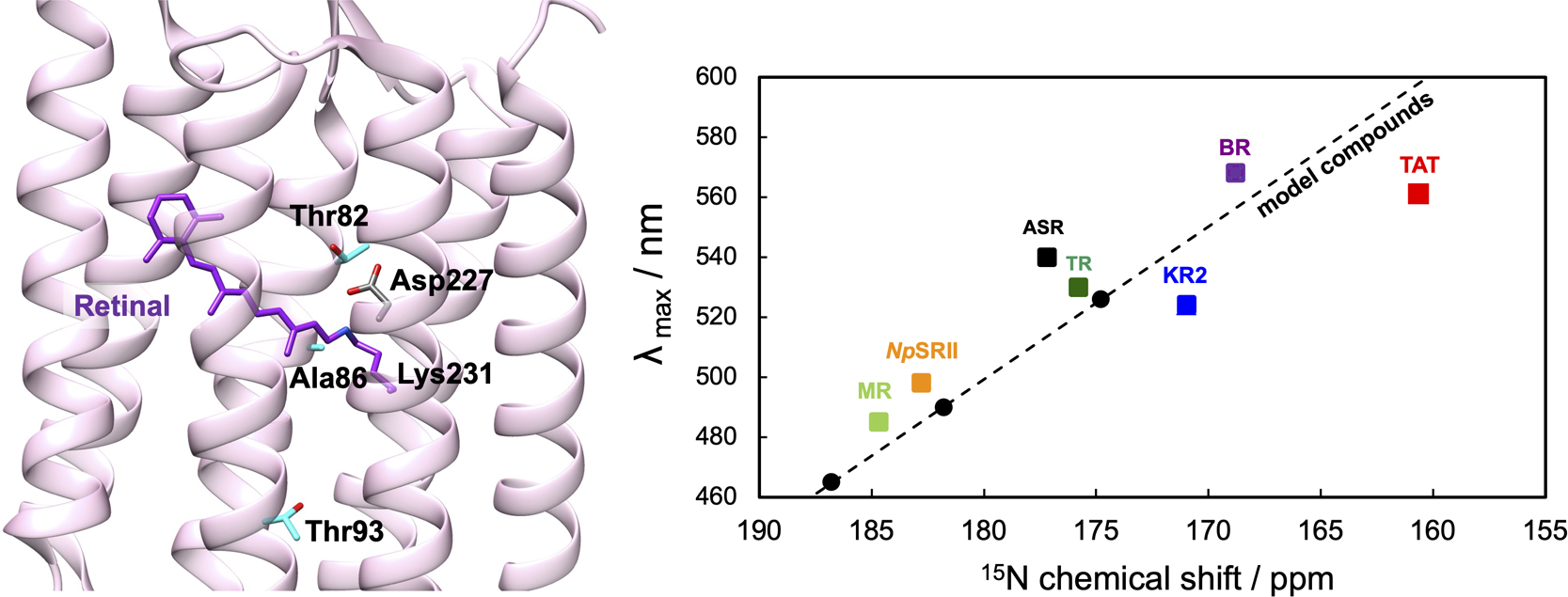
特集号: Biophysics and Physicobiology
20 巻 (2023)
- Supplemental 号
過去の巻号を選ぶ
20 巻, Supplemental 号
Special Issue: Recent Advances in Retinal Protein Research
選択された号の論文の23件中1~23を表示しています
- |<
- <
- 1
- >
- >|
Special Issue: Recent Advances in Retinal Protein Research
-
原稿種別: Special Issue: Recent Advances in Retinal Protein Research
2023 年 20 巻 Supplemental 号 論文ID: e201001
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2022/11/25PDF形式でダウンロード (305K) HTML形式で全画面表示
Commentary and Perspective (Memorial Address)
-
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective (Memorial Address)
2023 年 20 巻 Supplemental 号 論文ID: e201010
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/01/26PDF形式でダウンロード (699K) HTML形式で全画面表示
Commentary and Perspective
-
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201021
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/03/04PDF形式でダウンロード (369K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201022
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/03/07PDF形式でダウンロード (349K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201003
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/01/11PDF形式でダウンロード (864K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201014
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/02/21PDF形式でダウンロード (493K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201005
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/01/11PDF形式でダウンロード (1819K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201009
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/01/25PDF形式でダウンロード (551K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201012
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/02/09PDF形式でダウンロード (341K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201015
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/02/22PDF形式でダウンロード (455K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201018
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/03/02PDF形式でダウンロード (372K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201013
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/02/21PDF形式でダウンロード (500K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201019
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/03/02PDF形式でダウンロード (397K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201002
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2022/11/29PDF形式でダウンロード (519K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201020
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/03/02PDF形式でダウンロード (318K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Commentary and Perspective
2023 年 20 巻 Supplemental 号 論文ID: e201004
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2022/12/28PDF形式でダウンロード (416K) HTML形式で全画面表示
Review Article (Invited)
-
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Review Article (Invited)
2023 年 20 巻 Supplemental 号 論文ID: e201006
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/01/19PDF形式でダウンロード (2076K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Review Article (Invited)
2023 年 20 巻 Supplemental 号 論文ID: e201011
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/02/04PDF形式でダウンロード (1173K) HTML形式で全画面表示
Regular Article (Invited)
-
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Regular Article (Invited)
2023 年 20 巻 Supplemental 号 論文ID: e201008
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/02/03PDF形式でダウンロード (4186K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Regular Article (Invited)
2023 年 20 巻 Supplemental 号 論文ID: e201007
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/01/24PDF形式でダウンロード (3084K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Regular Article (Invited)
2023 年 20 巻 Supplemental 号 論文ID: e201017
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/03/02PDF形式でダウンロード (995K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Regular Article (Invited)
2023 年 20 巻 Supplemental 号 論文ID: e201016
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/02/25PDF形式でダウンロード (1903K) HTML形式で全画面表示 -
原稿種別: Special Issue: Recent Advances in Retinal Protein Research Regular Article (Invited)
2023 年 20 巻 Supplemental 号 論文ID: e201023
発行日: 2023年
公開日: 2023/03/21
[早期公開] 公開日: 2023/03/09PDF形式でダウンロード (5524K) HTML形式で全画面表示
- |<
- <
- 1
- >
- >|