
- |<
- <
- 1
- >
- >|
-
Hidetoshi Arima2017 年 65 巻 4 号 p. 311
発行日: 2017/04/01
公開日: 2017/04/01
ジャーナル フリー HTMLPDF形式でダウンロード (165K) HTML形式で全画面表示
-
Hatsuo Yamamura2017 年 65 巻 4 号 p. 312-317
発行日: 2017/04/01
公開日: 2017/04/01
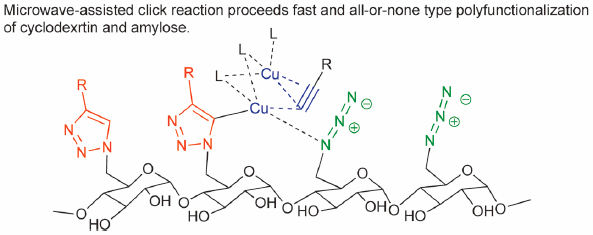
ジャーナル フリー HTMLCyclodextrin (CD) can be chemically modified into desired and sophisticated functional molecules. However, poly-modification often produces complicated mixtures, resulting in a low yield of the desired product. As the most promising procedure to solve such problems and to achieve poly-modification of the CD molecule, we present here the Huisgen 1,3-dipolar cycloaddition, known as a click reaction. This review will describe the results of our microwave-assisted click reaction for the poly-modification of CD and amylose molecules, and its application to the study of synthetic membrane active antibacterial derivatives.
 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (723K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (723K) HTML形式で全画面表示 -
Yuji Tsuchido, Shoji Fujiwara, Takeshi Hashimoto, Takashi Hayashita2017 年 65 巻 4 号 p. 318-325
発行日: 2017/04/01
公開日: 2017/04/01
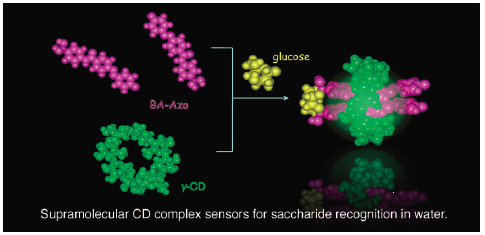
ジャーナル フリー HTMLCyclodextrins (CDs) are water-soluble host compounds having nano-size hydrophobic cavities that enable them to incorporate organic molecules in water. Optically inert CDs can be efficiently combined with various types of chromoionophores and fluoroionophores. In this study, using diverse combinations of phenylboronic acid fluorescent sensors and azoprobes with CDs, the unique saccharide recognition functions of CD, chemically modified CD, and CD gel complexes based on their synergistic function are clarified, thereby confirming their use as supramolecular saccharide sensors. To realize novel supramolecular chirality, the twisted structure of two ditopic azoprobes inside the γ-CD chiral cavity is controlled by multi-point recognition of guest ions in water. As different types of supramolecular saccharide sensors, phenylboronic acid-based self-assembling systems are also reviewed.
 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (2136K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (2136K) HTML形式で全画面表示 -
Kohzo Ito2017 年 65 巻 4 号 p. 326-329
発行日: 2017/04/01
公開日: 2017/04/01
ジャーナル フリー HTMLWe have recently synthesized slide-ring materials using cyclodextrin by cross-linking polyrotaxanes, a typical supramolecule. The slide-ring materials have polymer chains with bulky end groups topologically interlocked by figure-of-eight shaped junctions. This indicates that the cross-links can pass through the polymer chains similar to pulleys to relax the tension of the backbone polymer chains. The slide-ring materials also differ from conventional polymers in that the entropy of rings affects the elasticity. As a result, the slide-ring materials show quite small Young’s modulus not proportional to the cross-linking density. This concept can be applied to a wide variety of polymeric materials as well as gels. In particular, the slide-ring materials show remarkable scratch-proof properties for coating materials for automobiles, cell phones, mobile computers, and so on. Further current applications include vibration-proof insulation materials for sound speakers, highly abrasive polishing media, dielectric actuators, and so on.
 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (886K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (886K) HTML形式で全画面表示 -
Masaki Nakahata, Yoshinori Takashima, Akira Harada2017 年 65 巻 4 号 p. 330-335
発行日: 2017/04/01
公開日: 2017/04/01
ジャーナル フリー HTMLSmart design of polymeric materials may lead to intelligent materials exhibiting unique functional properties. Looking at nature, living systems use specific and reversible intermolecular interactions in realizing complex functions. Hence reversible bonds based on selective molecular recognition can impart artificial materials with unique functional properties. This review mainly focuses on supramolecular polymeric materials based on cyclodextrin-based host–guest interactions. Polymeric materials using molecular recognition at polymer main chain, side chain, and termini are described. Polymers carrying host and guest residues exhibit unique properties such as: 1) formation of macroscopic self-assembly of polymer gels carrying host and guest residues; 2) stimuli-responsive self-healing properties due to the reversible nature of host–guest interactions; and 3) macroscopic motion of artificial muscle cross-linked by host–guest interaction controlled by external stimuli. An overview of recent developments in this new frontier between materials science and life science is given.
 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (1476K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (1476K) HTML形式で全画面表示 -
Hiroaki Kitagishi, Saika Minegishi2017 年 65 巻 4 号 p. 336-340
発行日: 2017/04/01
公開日: 2017/04/01
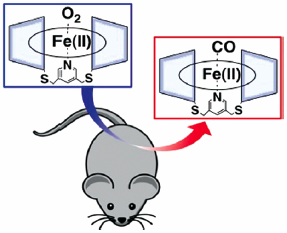
ジャーナル フリー HTMLThe specific intermolecular interaction between an anionic tetraarylporphyrin and per-O-methylated β-cyclodextrin (TMe-β-CD) paved the way to produce a functional supramolecule that works as a strong carbon monoxide (CO)-depleting agent in living organisms. The supramolecular complex, hemoCD, that is composed of meso-tetrakis(4-sulfonatophenyl)porphinatoiron(II) and a TMe-β-CD dimer linked by a pyridine linker, captured internal CO from carboxyhemoglobin during its circulation in the blood of animals. HemoCD thus produced the pseudo-knockdown (loss-of-functional) state of endogenous CO in the animals. This unique property led us to investigate the biological function of endogenous CO as a gaseous signal mediator in living systems. In this paper, we introduce our recent study on the hemoCD complex as a biological CO-depleting agent.
 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (712K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (712K) HTML形式で全画面表示 -
Hidetoshi Arima, Keiichi Motoyama, Taishi Higashi2017 年 65 巻 4 号 p. 341-348
発行日: 2017/04/01
公開日: 2017/04/01
ジャーナル フリー HTMLCyclodextrins (CyDs) are extensively used in various fields, and especially have been widely utilized as pharmaceutical excipients and drug carriers in the pharmaceutical field. Owing to the multi-functional and biocompatible characteristics, CyDs can improve the undesirable properties of drug molecules. This review outlines the current application of CyDs in pharmaceutical formulations, focusing on their use as CyD-based drug carriers for several kinds of drugs. Additionally, CyDs have great potential as active pharmaceutical ingredients against various diseases with few side effects.
 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (4889K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (4889K) HTML形式で全画面表示
-
Heejoo Park, Yeongji Yu, Hyejin Kim, Eun Lee, Hani Lee, Raok Jeon, Woo ...2017 年 65 巻 4 号 p. 349-355
発行日: 2017/04/01
公開日: 2017/04/01
[早期公開] 公開日: 2017/01/27ジャーナル フリー HTML
電子付録Many aminodihydroquinoline compounds have been studied to determine their cytotoxicity to cancer cells. However, anti-cancer stem cells (CSCs) activity of aminodihydroquinoline has not been tested in spite that CSC is believed to do an important roles in chemotherapy resistance and recurrence. The CSC selective targeting activities of 10 recently synthesized 2-aminodihydroquinoline analogs were examined on CSCs and bulk culture of a glioblastoma cell line. A diethylaminopropyl substituted aminodihydroquinoline, 5h, showed a strong anti-CSC effect and general cytotoxicity. However, a benzyl substituted aminodihydroquinoline, 5i, displayed the most effective anti-CSC effect, with no or small significant cytotoxic effect in bulk culture conditions. While 5h temporarily enhanced CSC marker-positive cells and eventually suppressed the CSC population, which is similar to other cytotoxic anticancer reagents reported, 5i selectively eliminated CSC marker-positive cells based on fluorescence activated cell sorter (FACS) analysis. 5h also temporarily activated some genes associated with signaling required for CSC, while 5i selectively suppressed these genes supporting that the differential effects are resulted from different molecular responses. In addition, the selective CSC effect is also found against a colon cancer cell line. Collectively, we suggest that these two novel aminodihydroquinoline compounds possess novel anti-CSC effects in colon and brain tumor derived cell lines probably through independent pathways.
 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (1505K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (1505K) HTML形式で全画面表示 -
 Takashi Okitsu, Take Matsuyama, Takahiro Yamashita, Toru Ishizuka, Hir ...2017 年 65 巻 4 号 p. 356-358
Takashi Okitsu, Take Matsuyama, Takahiro Yamashita, Toru Ishizuka, Hir ...2017 年 65 巻 4 号 p. 356-358
発行日: 2017/04/01
公開日: 2017/04/01
ジャーナル フリー HTML
電子付録Red-shifted channelrhodopsins (ChRs) are attractive for optogenetic tools. We developed a new type of red-shifted ChRs that utilized noncovalent incorporation of retinal and 3,4-dehydroretinal-based enamine-type Schiff bases and mutated channelopsin, C1C2-K296G. These ChRs exhibited absorption maxima that were shifted 10–30 nm toward longer wavelengths than that of C1C2-ChR regenerated with all-trans-retinal.
 Graphical Abstract Fullsize Image抄録全体を表示Editor's pick
Graphical Abstract Fullsize Image抄録全体を表示Editor's pickIn this communication, development of a new type of red-shifted channelrhodopsins (ChRs) is described. Optogenetics is a powerful new technique which allows control of neuronal activity by light, and ChRs are now widely used in optogenetics due to their function of a light-gated cation channel. In neuroscience, ChRs responding to a long-wavelength light are eagerly required, because ChRs now used are maximally sensitive to green and blue light, and does not penetrate tissues. Here developed new type of ChRs model consisted of red-shifted chromophores (retinal-based enamine-type Schiff bases) and mutated channelopsin (C1C2-K296G), in which chromophores were incorporated noncovalently. Thus prepared new ChRs exhibited absorption maxima that were 10-30 nm red-shifted compared with the original C1C2.
PDF形式でダウンロード (1118K) HTML形式で全画面表示
-
Kyoung Jin Park, Won Se Suh, Lalita Subedi, Sun Yeou Kim, Sang Un Choi ...2017 年 65 巻 4 号 p. 359-364
発行日: 2017/04/01
公開日: 2017/04/01
ジャーナル フリー HTML
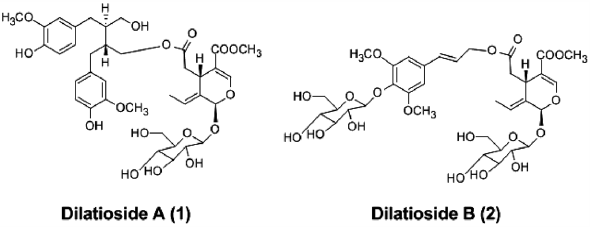
電子付録Phytochemical investigation of the twigs of Syringa oblata var. diatata led to the isolation of two new secoiridoid glucosides, dilatioside A–B (1–2), along with thirteen known ones (3–15). The structures were determined by spectroscopic methods including one and two dimensional (1- and 2D-) NMR techniques, high resolution (HR)-FAB-MS, and chemical methods. The isolated compounds (1–15) were tested for the induction of nerve growth factor (NGF) secretion in a C6 rat glioma cell line and their cytotoxicity against four human cancer cell lines (A549, SK-OV-3, SK-MEL-2, HCT15) in vitro using a sulforhodamine B bioassay. Compounds 5, 7, 8, 10, and 14 were found to induce upregulation of NGF secretion without causing significant cell toxicity.
 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (496K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (496K) HTML形式で全画面表示 -
Ryosuke Saijo, Hiroshi Sekiya, Eiji Tamai, Ken-ichi Kurihara, Jun Maki ...2017 年 65 巻 4 号 p. 365-372
発行日: 2017/04/01
公開日: 2017/04/01
ジャーナル フリー HTML
電子付録In this report, we describe a new method for the synthesis of densely functionalized 2(1H)-pyrazinones. Treatment of mesoionic 1,3-oxazolium-5-olates with carbanions derived from activated methylene isocyanides (p-toluenesulfonylmethyl isocyanide (TosMIC) and ethyl isocyanoacetate) causes a novel ring transformation affording 2(1H)-pyrazinones in moderate to high yields. The cytotoxicity and antibacterial activity of some of the obtained products were studied and some of the products exhibited tumor-specific cytotoxicity.
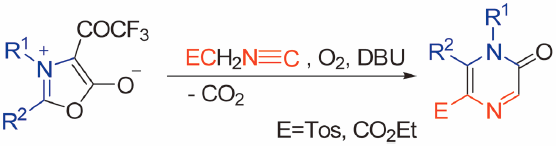 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (444K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (444K) HTML形式で全画面表示 -
Shinji Katsura, Nobuo Yamada, Atsushi Nakashima, Sumihiro Shiraishi, M ...2017 年 65 巻 4 号 p. 373-380
発行日: 2017/04/01
公開日: 2017/04/01
ジャーナル フリー HTMLWe observed that uncoated furosemide tablets turned yellow in a light-shielded automatic packaging machine and discoloration of the furosemide tablets was heterogeneity and occurred on the surface of the tablets only. The machine was equipped with an internal blower to maintain a constant temperature. Therefore, we investigated the effect of air flow on the discoloration of the furosemide tablets using a blower in a dark environment. The color difference (ΔE) of the furosemide tablets increased linearly as the blowing time increased. We performed structural analysis of the yellow compound in the furosemide tablets by LC-MS and identified the compound as a hydrolysate of furosemide. This suggested that furosemide hydrolysis was accelerated by the air flow. The furosemide tablets were prepared with the most stable furosemide polymorph, form I. X-Ray powder diffractometry and IR spectroscopy showed that during tablet preparation, no crystal transition occurred to an unstable furosemide polymorph. Furthermore, IR spectroscopy showed that the crystal form of furosemide in the yellow portion of the tablets was form I. To elucidate the factors producing the discoloration, we investigated the effect of humidity and atmosphere (air, oxygen, and nitrogen) on the discoloration of the furosemide tablets. The results suggested that the discoloration of the furosemide tablets was accelerated by oxidation, although humidity did not affect the hydrolysis. Therefore, we concluded that the discoloration of the furosemide tablets in the automatic packing machine was caused by acceleration of oxidative degradation by air flow.
 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (1520K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (1520K) HTML形式で全画面表示 -
Yingfei Wang, Xiaojing Zhang, Changjiang Xu, Gaoxiao Zhang, Zaijun Zha ...2017 年 65 巻 4 号 p. 381-388
発行日: 2017/04/01
公開日: 2017/04/01
ジャーナル フリー HTMLMyocardial ischemia is a primary cause of sudden death worldwide. Numerous active ingredients of traditional Chinese medicines including danshensu (DSS) and tetramethylpyrazine (TMP) have been widely used for the treatment of myocardial ischemia. To enhance their therapeutic efficacy and improve their drugability, in this work, we designed new DSS and TMP conjugates. Their water solubility and protective effects were studied in vitro and in experimental animal models. The new compounds demonstrated higher activities than the positive control agents acetylated danshensu and tetramethylpyrazine conjugate (ADTM) and salvianolic acid B (SAB) in preventing cells from oxidative insult. Among the new compounds, 14, bearing two glycine moieties, was more water soluble. In addition, compound 14 was much more potent in preventing cells from oxidative injury, at least 10- and 20-fold as potent as ADTM and SAB, respectively. The protective effects of compound 14 may be attributed to its anti-radical activity and anti-apoptotic activity. These results suggest that compound 14 is a promising candidate for the treatment of myocardial ischemia.
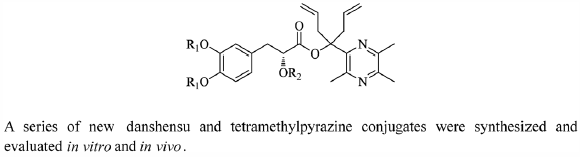 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (1545K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (1545K) HTML形式で全画面表示 -
Hitomi Uchimoto, Miki Ikeda, Saori Tanida, Kayo Ohhashi, Yoshiko Chiha ...2017 年 65 巻 4 号 p. 389-395
発行日: 2017/04/01
公開日: 2017/04/01
ジャーナル フリー HTML
電子付録We synthesize optically active (R)-terbutaline 2, which is an anti-asthmatic drug, through recyclable catalytic asymmetric transfer hydrogenation (RCATH). Various chloroketones 4 were prepared and RCATH was performed on them. The products exhibit moderate to high enantioselectivity. In particular, the hydrogenation of acyl substituted substrates 4c yields chiral secondary alcohols 5c in good yield and enantioselectivity. Furthermore, (R)-terbutaline 2 can be synthesized in one step from the resulting secondary alcohol 5 without racemization.
 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (671K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (671K) HTML形式で全画面表示 -
Takuro Yasuyama, Hirofumi Matsunaga, Shin Ando, Tadao Ishizuka2017 年 65 巻 4 号 p. 396-402
発行日: 2017/04/01
公開日: 2017/04/01
[早期公開] 公開日: 2017/02/04ジャーナル フリー HTMLA novel type of molecularly imprinted polymer (MIP), N-benzoyl-(S)-valine anilide-imprinted polymer (IP-2), was prepared using hydrogen-bonding interactions as a main force in the pre-polymerization step. The performance of the IP-2 was evaluated via batch procedure and compared with a (S)-valine anilide-imprinted polymer (IP-1) that was prepared using an ionic interaction that is stronger than hydrogen bonding. Although both polymers showed a preferential adsorbability for (S)-amino acid derivatives, different performances were observed in terms of adsorbability and enantioselectivity. In addition, the IP-2 was able to recognize the enantiomer of a valine-derived chiral catalyst. This phenomenon was applied to a chiral amplification reaction, and a highly selective asymmetric Mannich-type amination was achieved using the combination of a racemic catalyst and a MIP.
 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (658K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (658K) HTML形式で全画面表示
-
Hong-Mei Li, Min Fan, Yong Xue, Li-Yan Peng, Xing-De Wu, Dan Liu, Rong ...2017 年 65 巻 4 号 p. 403-407
発行日: 2017/04/01
公開日: 2017/04/01
ジャーナル フリー HTML
電子付録Twelve guaiane-type sesquiterpenoids, including four new ones, 10-O-methyl-orientalol A (1), 10-O-ethyl-alismoxide (2), 3β,4β-expoxy-chrysothol (3), orientalol G (4), and a new norsesquiterpenoid, orientalol H (5), were isolated from Chinese Alismatis Rhizoma, the dried rhizome of Alisma orientale. The structures of new compounds were elucidated on the basis of spectroscopic data. Moreover, the inhibition of lipopolysaccharide (LPS)-induced nitric oxide (NO) production in RAW264.7 cells of all compounds was tested.
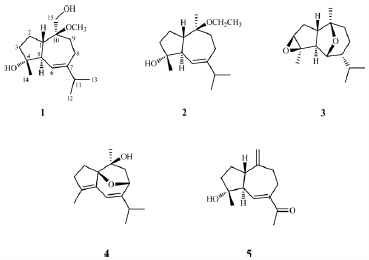 Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (492K) HTML形式で全画面表示
Graphical Abstract Fullsize Image抄録全体を表示PDF形式でダウンロード (492K) HTML形式で全画面表示
- |<
- <
- 1
- >
- >|