
- |<
- <
- 1
- >
- >|
-
 Takuya Miura, Hidetoshi Fujii, Kohsaku UshiodaArticle type: Regular Article
Takuya Miura, Hidetoshi Fujii, Kohsaku UshiodaArticle type: Regular Article
2022 Volume 86 Issue 6 Pages 87-96
Published: June 01, 2022
Released on J-STAGE: May 25, 2022
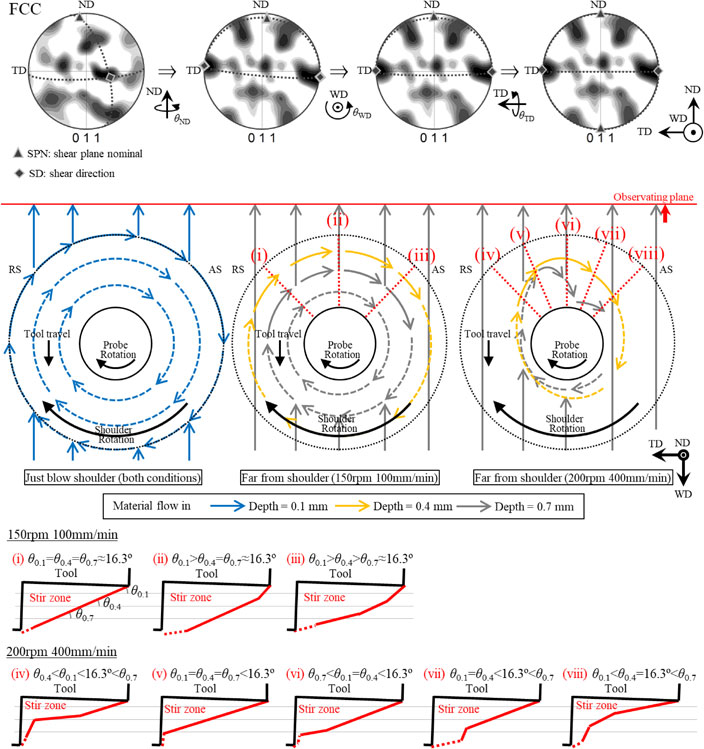
Advance online publication: April 08, 2022JOURNAL FREE ACCESS FULL-TEXT HTMLFriction stir welding (FSW) was performed under the two welding conditions (rotation speed-traveling speed) of 150 rpm-100 mm/min and 200 rpm-400 mm/min using 6 mass%Ni-0.63 mass%C steels. The slightly lower peak welding temperature and significantly higher cooling rate was obtained under the condition of 200 rpm-400 mm/min. Texture analysis for retained austenite and martensite revealed that the parent austenite had simple shear texture, which lead to the formation of {110}<111> texture in transformed martensite. Moreover, material flow behavior as a function of distance from the top surface in the stir zone was analyzed based on textures obtained by EBSD measurement. A concentric material flow, which has smaller radius as the far from the tool shoulder, was formed under the condition of 150 rpm-100 mm/min. On the other hand, a heterogeneous material flow with the center shifted to the retreating side was formed under the condition of 200 rpm-400 mm/min. In addition, a different vertical component of material flow was predicted to occur under each welding conditions.
 View full abstractEditor's pick
View full abstractEditor's pickYoung Author Best Paper Award 2023
Download PDF (9682K) Full view HTML -
Kohei Mori, Yuta Yamakawa, Satoshi Oue, Yu-ki Taninouchi, Hiroaki Naka ...Article type: Regular Article
2022 Volume 86 Issue 6 Pages 97-106
Published: June 01, 2022
Released on J-STAGE: May 25, 2022
Advance online publication: April 08, 2022JOURNAL FREE ACCESS FULL-TEXT HTMLThe Cu electrorefining using low-grade copper anode is desired from the view point of reduction of electric power. Cu electrolysis was performed using a low-grade copper anode in an unagitated sulfate solution, and the effect of impurity ions and additives in solution on the passivation of anode was investigated. The time when anode passivation firstly occurs shortened significantly in solution containing 0.596 mol·dm−3 of Ni2+ ions as impurity, and shortened somewhat in solution containing As5+(0.053 mol·dm−3) or Bi3+(0.0005 mol·dm−3) ions. Sn2+(0.0004 mol·dm−3) and As3+(0.053 mol·dm−3) ions slightly decreased the time to passivation, but Sb3+ (0.004 mol·dm−3) ions rarely decreased the time. When the 0.596 mol·dm−3 of Ni2+ ions were added in solution, the viscosity coefficient of solution increased and the diffusion coefficient of Cu2+ ions decreased. When the As5+(0.053 mol·dm−3) or Bi3+(0.0005 mol·dm−3) ions were added in solution, the compound of As-Sb-O or As-Bi-O system was formed in anode slime, which seemed to increase the compactness of slime. The time to passivation was slightly longer in thiourea-free solution, but shortened with increasing the concentration of thiourea from 0.525 to 2.24 mmol·dm−3. In the solutions containing 0 to 1.13 mmol·dm−3 of Cl− ions, the time to passivation was constant, but significantly shortened with increasing the concentration of Cl− ions at the region above 1.13 mmol·dm−3. Cl− ions formed CuCl at the upper area of anode slime, as a result, increased the compactness of slime and promoted the passivation.
 Fig. 2 Time dependence of anode potential during electrolysis at 200 A·m−2 in the solutions containing various kinds of impurities. [Additive: Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Cl− (1.13 mmol·dm−3), Impurity: Ni2+(0.596 mol·dm−3), As5+(0.053 mol·dm−3), Bi3+(0.0005 mol·dm−3), Sn2+(0.0004 mol·dm−3), As3+(0.053 mol·dm−3), Sb3+ (0.004 mol·dm−3)] Fullsize ImageView full abstractDownload PDF (6237K) Full view HTML
Fig. 2 Time dependence of anode potential during electrolysis at 200 A·m−2 in the solutions containing various kinds of impurities. [Additive: Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Cl− (1.13 mmol·dm−3), Impurity: Ni2+(0.596 mol·dm−3), As5+(0.053 mol·dm−3), Bi3+(0.0005 mol·dm−3), Sn2+(0.0004 mol·dm−3), As3+(0.053 mol·dm−3), Sb3+ (0.004 mol·dm−3)] Fullsize ImageView full abstractDownload PDF (6237K) Full view HTML
- |<
- <
- 1
- >
- >|