
- Issue 6 Pages 263-
- Issue 5 Pages 203-
- Issue 4 Pages 159-
- Issue 3 Pages 113-
- Issue 2 Pages 63-
- Issue 1 Pages 1-
- |<
- <
- 1
- >
- >|
-
Tohru KamoArticle type: Review Paper
2017 Volume 60 Issue 2 Pages 63-71
Published: March 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSIn normal resource recovery processes, the manually dismantled waste products are pulverized and sorted by using differences in specific gravity. However, new technology using chemical reactions is required to recover useful materials from composite wastes such as printed circuit boards and carbon fiber reinforced plastics. Our research group has been developing three material recovery technologies employing hydrogen donor solvents, recycled solvents derived from biomass, and low temperature steam gasification with catalysts. A liquefaction method using partially hydrogenated aromatic compounds as a solvent liquefied resol phenol, which is hard to liquefy using other technologies, and could produce clean fuel that contains almost no toxic organic chlorine compounds from polyvinyl chloride. The use of a liquefaction method employing an ester exchange reaction is limited to certain types of plastics, such as epoxy resin, but thermosetting resins can be liquefied under mild conditions and atmospheric pressure with this technology. We also found that solvents can be produced from biomass or previously liquefied plastics. Steam gasification with catalysts can be applied to all organic materials such as plastics, wood, and rubber. Furthermore, the technology is very energy efficient because the operational temperature is much lower than that of conventional gasification.
 View full abstractDownload PDF (3608K)
View full abstractDownload PDF (3608K) -
Shun Nishimura, Kohki EbitaniArticle type: Review Paper
2017 Volume 60 Issue 2 Pages 72-84
Published: March 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSSimple transformation of sustainable biomass-resources to value-added chemicals and fuels has become important to diminish our heavy reliance on decreasing petroleum resources. Biomass-derived materials with hydroxyl groups such as 5-hydroxymethylfurfural (HMF) and glycerol can be obtained from sugars directly and transesterification of triglycerides with methanol as by-products, respectively. These chemicals are versatile starting materials because hydroxyl groups can be easily converted to afford value-added chemicals and fuels under oxidative/reductive conditions using supported metal catalysts. This review overviews recent studies on supported metal catalyzed oxidation reactions of HMF, glycerol and biomass-derived aliphatic α,ω-diols together with our research to develop highly efficient catalytic systems for bio-refining, based on petroleum conversion and nano-technologies. Especially, role of external bases in the selective oxidation of biomass-derived alcohols will be discussed. We found that external base-free oxidation of HMF and glycerol is possible if basic supports are used. The activity of alloyed metal nanoparticles for selective oxidation of aliphatic α,ω-diols to ω-hydroxycarboxylic acids in basic aqueous media at high-pH value is also demonstrated.
 View full abstractDownload PDF (1463K)
View full abstractDownload PDF (1463K)
-
Goshtasp CheraghianArticle type: Regular Paper
2017 Volume 60 Issue 2 Pages 85-94
Published: March 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSStatic and dynamic adsorption have key role in chemical flooding process and they are important parameters in surfactant polymer degradation and decrease oil recovery. The effects of nano concentration on static adsorption of surfactant were investigated at variable condition polymer and surfactant concentration and nanoparticles are critical parameters influence the adsorption behavior at a flooding process. Surfactant polymer solutions and newly developed nanoparticles solutions were tested. The crude oil had a viscosity of 1320 mPa s at test conditions. In this paper, the role of nanoparticles in the adsorption of surfactant polymers onto solid surfaces of reservoir core is studied. The results which obtained by means of static adsorption tests, show that the adsorption is dominated by the clay and silica nanoparticles between the polymer molecules and the solid surface. Higher nanoparticles concentration leads to less adsorption, where the adsorption may decrease to 20 % of the adsorption level of surfactant polymer. The clay and Aerosil A300 nanoparticles in surfactant polymer solutions improved oil recovery by about the same amount. The clay, however, showed improved performance in comparison to Aerosil A300.
 View full abstractDownload PDF (1075K)
View full abstractDownload PDF (1075K) -
Yusuke Kawahara, Kazuki Komiyama, Kenji AsamiArticle type: Regular Paper
2017 Volume 60 Issue 2 Pages 95-100
Published: March 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSThe Fischer-Tropsch synthesis (FTS) was conducted for active-carbon-supported iron catalysts in a slurry-phase reactor. Heat treatment of the active carbon support in N2 resulted in some improvement in catalytic durability. However, ozone (O3) treatment of the support greatly improved catalytic durability. The optimum treatment temperature was found to be 400 °C. X-ray diffraction measurements and N2 adsorption analysis revealed that the structure of the active carbon support did not change drastically by the O3 treatment but the average pore size and mesopore volume increased to 1.3 times of those of the untreated support. This suggests that O3 removes a part of the amorphous carbon phase, thereby enlarging the pores to some extent. The major reason for catalyst deactivation was assumed to be the blocking of active sites by waxy products that formed. The enlarged pores facilitated solvent extraction from heavier products and thus promoted the maintenance of high catalyst activity. The product distribution obtained from the O3-treated catalyst exhibited a higher proportion of low-carbon-number products, thereby indicating suppression of secondary chain growth.
 View full abstractDownload PDF (994K)
View full abstractDownload PDF (994K) -
Pattasuda Duangkaew, Shuhei Inoue, Tsunehiro Aki, Yutaka Nakashimada, ...Article type: Regular Paper
2017 Volume 60 Issue 2 Pages 101-109
Published: March 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSQuantitative in-situ analysis by mass spectroscopic (MS) method was developed and designed in order to elucidate the decomposition kinetics of glucose under hydrothermal conditions. Briefly, the proposed methodology employed a quadrupole mass analyzer coupled with a tubular batch reactor via custom-built connection fittings. At first, the hydrothermal treatment of glucose was conducted at temperatures ranging from 140 to 220 °C with reaction times between 2.5 min and 20 min. Liquid product obtained after the hydrothermal process was quantitatively analyzed with a mass analyzer and a high-performance liquid chromatography (HPLC) analyzer. The in-situ MS analysis revealed that the decomposition rate of glucose was enhanced at higher temperatures. The results also showed the presence of 5-hydroxymethylfurfural and other low-molecular-weight acids whose concentrations increased when the temperature increased to 180 °C and higher. A reaction pathway of glucose was developed, and the reaction rate constants were determined assuming first-order reactions.
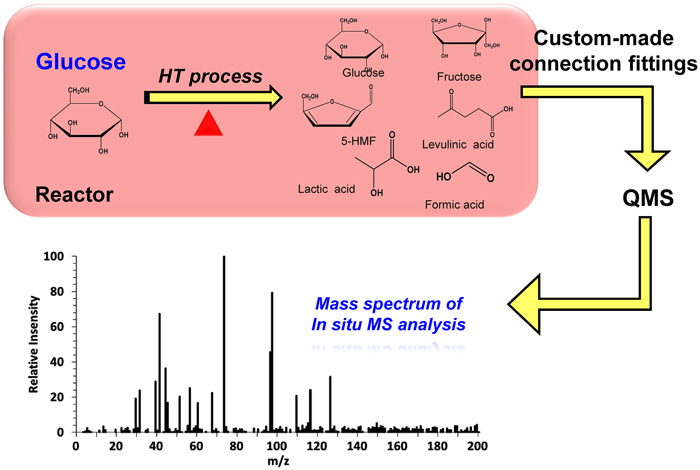 View full abstractDownload PDF (946K)
View full abstractDownload PDF (946K)
-
Yoshio Nishimura, Masaki Sakon, Masato TakiArticle type: Letter
2017 Volume 60 Issue 2 Pages 110-111
Published: March 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSFree sulfur contained in the oily wastewater (bilge) at the bottom of a ship often dissolves once discharged into the sea. Such changes in free sulfur content may prevent accurate matching of the bilge discharged into the sea with the bilge of the responsible ship. This study examined the behavior of free sulfur in seawater to test the hypothesis that microorganisms in seawater contribute to dissolving free sulfur. This study also used a method for quickly removing free sulfur by solid-phase extraction to examine the dissolution of free sulfur in seawater.
 View full abstractDownload PDF (935K)
View full abstractDownload PDF (935K)
- |<
- <
- 1
- >
- >|