
- |<
- <
- 1
- >
- >|
-
Tetsuaki Fujihara, Yasushi TsujiArticle type: Review Paper
2018Volume 61Issue 1 Pages 1-9
Published: January 01, 2018
Released on J-STAGE: January 01, 2018
JOURNAL FREE ACCESSVarious available approaches for catalytic carbonylation reactions using formates and formamides with transition metal catalysts are reviewed. Pd complexes catalyze the hydroesterification or hydrocarbamoylation of alkynes using aryl formates or formamides, respectively, as the carbonyl sources. The esterification of aryl halides with aryl formates proceeds in the presence of a Pd catalyst with suitable ligands. Using a Cu complex with a bulky bidentate phosphine ligand is used as a catalyst, boraformylation and silaformylation of allenes efficiently proceeds using alkyl formates as a formyl source.
 View full abstractDownload PDF (540K)
View full abstractDownload PDF (540K)
-
Kohei Kubo, Hajime Iida, Seitaro Namba, Akira IgarashiArticle type: Review Paper
2018Volume 61Issue 1 Pages 10-19
Published: January 01, 2018
Released on J-STAGE: January 01, 2018
JOURNAL FREE ACCESSCatalytic cracking of C6-8 hydrocarbons as model naphtha components was evaluated over H-ZSM-5 catalysts with various Si/Al ratios at 723-923 K to selectively form light olefins. The highest ethylene + propylene yield was 59.7 C-% with a propylene/ethylene ratio of ca. 0.72 at 99.6 % conversion in the cracking of n-heptane over H-ZSM-5 (Si/Al = 31) at 923 K. The selectivities and activation energies indicated that monomolecular cracking was predominant at temperatures higher than 923 K. Next, cracking of various C6-8 hydrocarbons on H-ZSM-5 at high temperatures in the presence of another hydrocarbon was also investigated. Coexistent 1-hexene did not affect the cracking rate of n-heptane, cyclohexane, or methylcyclohexane, also indicating that monomolecular cracking was predominant at temperatures as high as 923 K. In contrast, coexistent cyclohexane and methylcyclohexane considerably affected the cracking rate of n-heptane because of their slow diffusion in the pores of H-ZSM-5. Moreover, catalysts with activity equal or higher than that of H-ZSM-5 and steaming stability higher than that of H-ZSM-5 were examined. The 194 %Cu-ZSM-5 and 95 %Ag-ZSM-5 zeolites exhibited high steaming stabilities and higher cracking activities than H-ZSM-5 and P/H-ZSM-5. In particular, 194 %Cu-ZSM-5 exhibited an ultra-high steaming stability under conditions such as 1023 K and 10 h presumably because Cu2+ and Cu+ cations in the 194 %Cu-ZSM-5 are not reduced to Cu0 at a high temperature of 1023 K in the presence of O2.
 View full abstractDownload PDF (674K)
View full abstractDownload PDF (674K) -
Atsushi Takahashi, Tadahiro FujitaniArticle type: Review Paper
2018Volume 61Issue 1 Pages 20-27
Published: January 01, 2018
Released on J-STAGE: January 01, 2018
JOURNAL FREE ACCESSThe ethanol conversion reaction was systematically investigated over ZSM-5 zeolite catalysts with various Si/Al2 ratios (73, 128, 176, 207) using different contact times and reaction temperatures. The reaction yielded similar product distributions over all catalysts at different contact times, indicating that the product distribution has no relationship with the Si/Al2 ratio. Catalytic activity was proportional to the number of Brønsted acid sites. The mechanism of ethanol conversion was independent of the Si/Al2 ratio and propylene was directly produced via ethylene. Mechanistic analysis of the initial stage of the reaction suggested that ethylene was transformed to a carbene, which was a transient intermediate in the direct production of propylene. ZSM-5 zeolite was modified with phosphorus to improve propylene selectivity. Phosphorus-modified ZSM-5 catalysts showed enhanced selectivity for propylene production and good catalytic stability.
 View full abstractDownload PDF (1376K)
View full abstractDownload PDF (1376K) -
Takayuki KomatsuArticle type: Review Paper
2018Volume 61Issue 1 Pages 28-36
Published: January 01, 2018
Released on J-STAGE: January 01, 2018
JOURNAL FREE ACCESSCoke formation was investigated in the catalytic cracking of alkanes into light alkenes over ferrierite and ZSM-5 zeolites. H-ferrierite had the highest alkene selectivity in heptane cracking because of its smaller pore size. Ca2+-exchange into H-ferrierite improved the selectivity and controlled coke formation. Ca2+ ions located at the center of the 8-membered ring converted ferrierite pores into one-dimensional channels of 10-membered rings, which suppressed the bimolecular hydride transfer to form alkanes and coke precursors. H-ZSM-5 zeolites having various extents of coke deposited in hexane cracking were characterized by adsorption measurements of various alkane molecules. Small amounts of coke did not affect the hexane conversion and micropore volume, whereas the adsorption rate of 2,3-dimethylbutane decreased significantly. These results suggest that coke is accumulated on the external surface of H-ZSM-5. Most coke would be formed via aromatic hydrocarbons. Selectivity for the transformation of aromatics into coke correlated with crystallite size of H-ZSM-5, indicating that aromatics formed in short channels can diffuse out of the crystallite immediately without coke precursor formation. H-ZSM-5 with smaller crystallite size is likely to be a stable catalyst for naphtha cracking into light alkenes with adequate catalyst life.
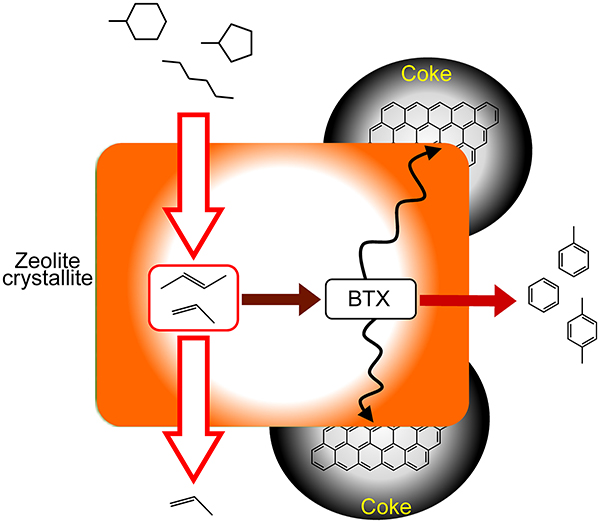 View full abstractDownload PDF (1499K)
View full abstractDownload PDF (1499K)
-
Hideaki MikiArticle type: Regular Paper
2018Volume 61Issue 1 Pages 37-43
Published: January 01, 2018
Released on J-STAGE: January 01, 2018
JOURNAL FREE ACCESSThe deactivation factors of a Pd/Al2O3 catalyst in the selective hydrogenation process of cyclopentadiene (CPD) to cyclopentene (CPE) were investigated with using continuance flow system equipped with bench scale reactor (Mono-Tube Reactor) in order to develop effective manufacturing process of CPE. The position of maximum temperature in the reactor (so call Hot Spot) gradually moved from the inlet to the outlet of the reactor and the Hot Spot disappeared when time on stream (T.O.S.) reached 100 h. Here, the deactivation factors of the catalyst before and after the disappearance of Hot Spot were discussed on the basis of the examinations of the reaction operating after replacing to the new catalyst and with using high purity materials.
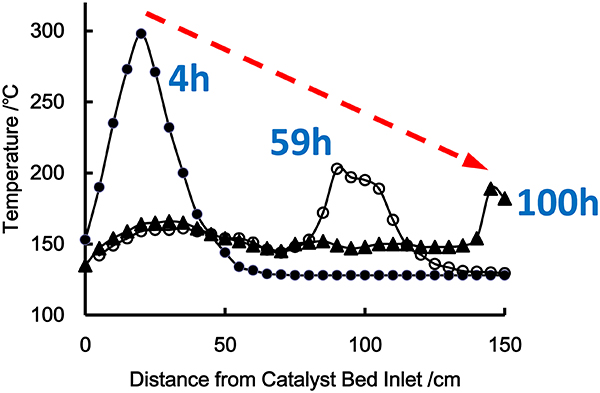 View full abstractDownload PDF (1021K)
View full abstractDownload PDF (1021K) -
Masaki Kogiso, Masaru AoyagiArticle type: Regular Paper
2018Volume 61Issue 1 Pages 44-49
Published: January 01, 2018
Released on J-STAGE: January 01, 2018
JOURNAL FREE ACCESSA novel treatment technique for produced water was developed using organic nanotubes (ONTs) as adsorbents. ONTs formed by the self-assembly of peptide lipids are known to adsorb heavy metals and hydrophobic molecules. Preferential adsorption of heavy metals against alkali- and alkali-earth metals above pH 6 was confirmed. Pb2+ in high-salinity model produced water was reduced to 0.04 ppm by treatment with ONTs, compared to 0.84 ppm without ONTs. The selectivity of Pb2+ against alkali-earth metals was over 3000. Design of the molecular structures of peptide lipids reduced the content of p-cresol in model produced water from 13 to less than 1 ppm. To improve separation from wastewater, ONTs hybridized with magnetic nanoparticles (MNPs) were obtained through the pH adjusting method. Hybrids maintained the hybridized state over a wide pH range (1-9). Adsorption tests using real produced water indicated that ONTs could adsorb heavy metals, suspended solids, oil, and some organic compounds in produced water in a one-step process, without pre-treatment.
 View full abstractDownload PDF (1290K)
View full abstractDownload PDF (1290K)
- |<
- <
- 1
- >
- >|