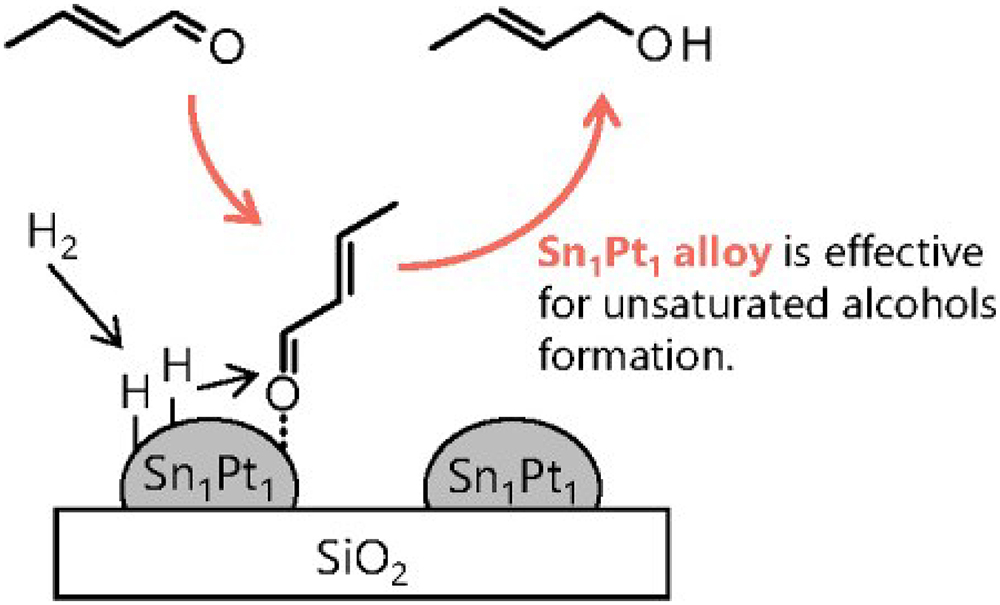
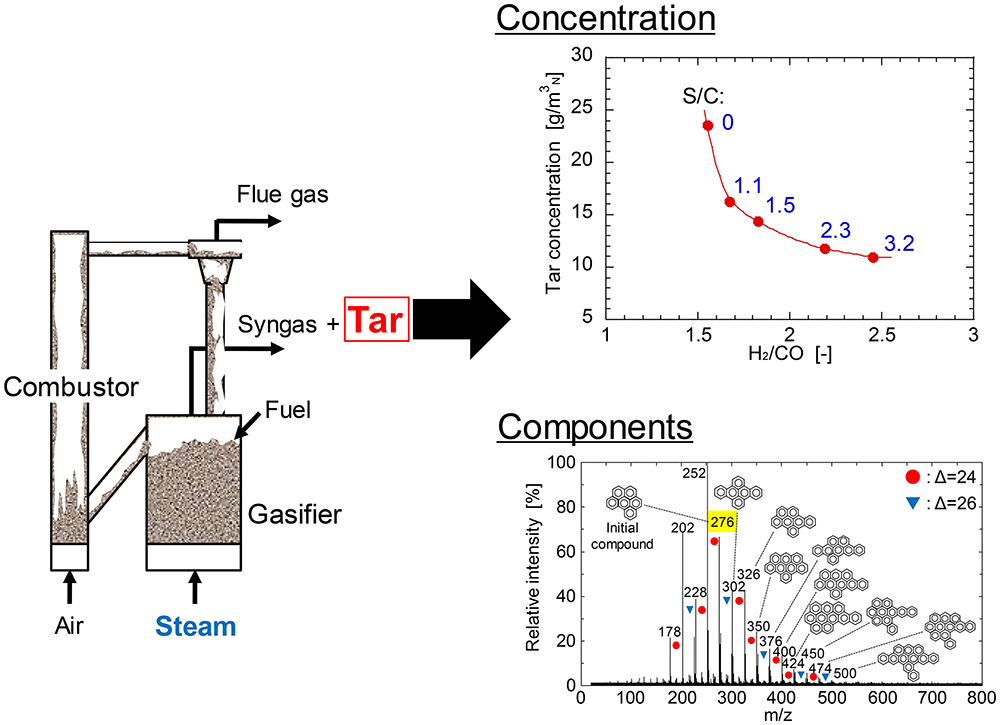
Volume 63, Issue 2
Displaying 1-8 of 8 articles from this issue
- |<
- <
- 1
- >
- >|
Review Paper
-
Article type: Review Paper
2020 Volume 63 Issue 2 Pages 43-51
Published: March 01, 2020
Released on J-STAGE: March 01, 2020
Download PDF (2367K) -
Article type: Review Paper
2020 Volume 63 Issue 2 Pages 52-61
Published: March 01, 2020
Released on J-STAGE: March 01, 2020
Download PDF (1694K)
Regular Paper
-
Article type: Regular Paper
2020 Volume 63 Issue 2 Pages 62-69
Published: March 01, 2020
Released on J-STAGE: March 01, 2020
Download PDF (3771K) -
Article type: Regular Paper
2020 Volume 63 Issue 2 Pages 70-78
Published: March 01, 2020
Released on J-STAGE: March 01, 2020
Download PDF (1524K) -
Article type: Regular Paper
2020 Volume 63 Issue 2 Pages 79-88
Published: March 01, 2020
Released on J-STAGE: March 01, 2020
Download PDF (5931K) -
Article type: Regular Paper
2020 Volume 63 Issue 2 Pages 89-95
Published: March 01, 2020
Released on J-STAGE: March 01, 2020
Download PDF (5176K) -
Article type: Regular Paper
2020 Volume 63 Issue 2 Pages 96-101
Published: March 01, 2020
Released on J-STAGE: March 01, 2020
Download PDF (1150K)
Letter
-
Article type: Letter
2020 Volume 63 Issue 2 Pages 102-105
Published: March 01, 2020
Released on J-STAGE: March 01, 2020
Download PDF (506K)
- |<
- <
- 1
- >
- >|