
- Issue 16 Pages 2157-
- Issue 15 Pages 1805-
- Issue 14 Pages 1613-
- Issue 13 Pages S1141-
- Issue 12 Pages S1037-
- Issue 11 Pages 1443-
- Issue 10 Pages 1273-
- Issue 9 Pages 1077-
- Issue 8 Pages 917-
- Issue 7 Pages 751-
- Issue 6 Pages 585-
- Issue 5 Pages S389-
- Issue 4 Pages S1-
- Issue 3 Pages 403-
- Issue 2 Pages 233-
- Issue 1 Pages 19-
- Issue 16 Pages 2153-
- Issue 15 Pages 1977-
- Issue 14 Pages 1813-
- Issue 13 Pages S1285-
- Issue 12 Pages S1043-
- Issue 11 Pages 1667-
- Issue 10 Pages 1481-
- Issue 9 Pages 1231-
- Issue 8 Pages 891-
- Issue 7 Pages 711-
- Issue 6 Pages 538-
- Issue 5 Pages S407-
- Issue 4 Pages S1-
- Issue 3 Pages 347-
- Issue 2 Pages 173-
- Issue 1 Pages 14-
- Issue 16 Pages 1837-
- Issue 15 Pages 1711-
- Issue 14 Pages 1569-
- Issue 13 Pages S1205-
- Issue 12 Pages S1034-
- Issue 11 Pages 1423-
- Issue 10 Pages 1269-
- Issue 9 Pages 1059-
- Issue 8 Pages 925-
- Issue 7 Pages 775-
- Issue 6 Pages 627-
- Issue 5 Pages S287-
- Issue 4 Pages S1-
- Issue 3 Pages 301-
- Issue 2 Pages 147-
- Issue 1 Pages 12-
- Issue 16 Pages 2179-
- Issue 15 Pages 1795-
- Issue 14 Pages 1631-
- Issue 13 Pages S1053-
- Issue 12 Pages S1023-
- Issue 11 Pages 1501-
- Issue 10 Pages 1315-
- Issue 9 Pages 987-
- Issue 8 Pages 767-
- Issue 7 Pages 621-
- Issue 6 Pages 473-
- Issue 5 Pages S305-
- Issue 4 Pages S1-
- Issue 3 Pages 299-
- Issue 2 Pages 151-
- Issue 1 Pages 16-
- Issue 16 Pages 1945-
- Issue 15 Pages 1699-
- Issue 14 Pages 1531-
- Issue 13 Pages S1055-
- Issue 12 Pages S1013-
- Issue 11 Pages 1367-
- Issue 10 Pages 1215-
- Issue 9 Pages 1087-
- Issue 8 Pages 887-
- Issue 7 Pages 721-
- Issue 6 Pages 507-
- Issue 5 Pages S317-
- Issue 4 Pages S1-
- Issue 3 Pages 343-
- Issue 2 Pages 187-
- Issue 1 Pages 17-
- Issue 16 Pages 2405-
- Issue 15 Pages 2067-
- Issue 14 Pages 1865-
- Issue 13 Pages 1675-
- Issue 12 Pages S1055-
- Issue 11 Pages S1015-
- Issue 10 Pages 1479-
- Issue 9 Pages 1129-
- Issue 8 Pages 895-
- Issue 7 Pages 711-
- Issue 6 Pages 545-
- Issue 5 Pages S325-
- Issue 4 Pages S1-
- Issue 3 Pages 369-
- Issue 2 Pages 193-
- Issue 1 Pages 16-
- Issue 16 Pages 2573-
- Issue 15 Pages 2261-
- Issue 14 Pages 2073-
- Issue 13 Pages S1111-
- Issue 12 Pages S1001-
- Issue 11 Pages 1867-
- Issue 10 Pages 1657-
- Issue 9 Pages 1409-
- Issue 8 Pages 1043-
- Issue 7 Pages 841-
- Issue 6 Pages 649-
- Issue 5 Pages S415-
- Issue 4 Pages S1-
- Issue 3 Pages 431-
- Issue 2 Pages 225-
- Issue 1 Pages 3-
- |<
- <
- 1
- >
- >|
-
2019 Volume 105 Issue 7 Pages Cover-
Published: July 01, 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESSDownload PDF (666K) -
2019 Volume 105 Issue 7 Pages Contents-
Published: July 01, 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESSDownload PDF (566K) -
2019 Volume 105 Issue 7 Pages Editorial-
Published: July 01, 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESSDownload PDF (230K)
-
Naoki Takata, Ryosuke Kainuma2019 Volume 105 Issue 7 Pages 675
Published: 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESS FULL-TEXT HTMLDownload PDF (245K) Full view HTML
-
Yusuke Okumura, Minoru Tanaka, Yusuke Fushiwaki, Yasunobu Nagataki2019 Volume 105 Issue 7 Pages 676-682
Published: 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESS FULL-TEXT HTMLIn the process of hot-dip galvanizing of steel, alloying elements such as Si and Mn are easily oxidized by H2O in the annealing atmosphere, causing coating defects. Because this selective oxidation depends on the annealing heat pattern, i.e., the soaking temperature and time, basic research on the kinetics of selective oxidation is important for clarifying the phenomenon of selective oxidation. In this study, the effects of the annealing temperature and dew point on the kinetics and compounds of Mn external oxidation were investigated experimentally, and the Mn external oxidation rate was estimated based on a diffusion equation and thermodynamic equilibrium, considering the diffusion coefficient and the activity coefficient of Mn in steel. The amount of Mn oxide increased in proportion to the square root of the soaking time. This result suggests that Mn oxidation is a diffusion limited process. The Mn oxidation rate increased with increasing temperature and reached a peak value, and at higher temperatures, the Mn oxidation rate became dramatically slower. The peak value also depended on the dew point. To clarify the reason for this slowdown of Mn oxidation, the Mn oxidation rate was estimated. Considering the activity coefficient and the diffusion coefficient of Mn in steel, the calculated Mn oxidation rate was consistent with the measured value. It is thought that the Mn oxidation rate slows at high temperature because the gradient of the Mn concentration around the steel surface becomes small at high temperatures near the equilibrium temperature of Mn/MnO.
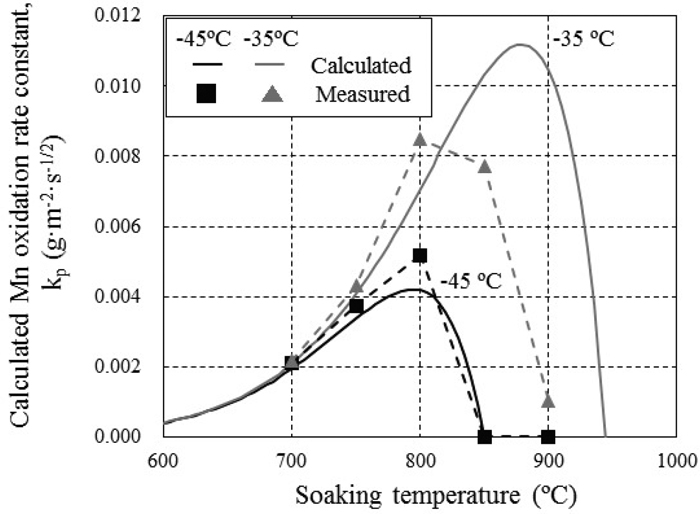 View full abstractDownload PDF (2434K) Full view HTML
View full abstractDownload PDF (2434K) Full view HTML -
Mai Miyata, Yusuke Fushiwaki, Yoshitsugu Suzuki, Hideki Nagano, Yasuno ...2019 Volume 105 Issue 7 Pages 683-692
Published: 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESS FULL-TEXT HTMLThe influence of the Si/Mn ratio on the galvannealing behavior of 1.5 wt% Si -1.5~2.5 wt% Mn-added steel in the Fe oxidation-reduction process was investigated. The Si/Mn ratio of the steel affected the formation of Si-containing oxides during the annealing process. The amount of SiO2 formed on the steel surface decreased with as the Si/Mn ratio decreased, while the amount of Mn2SiO4 increased. In addition, the internal oxide formed in a relatively narrow area near the surface in the lower Si/Mn ratio sample, which indicated that the content of solute Si near the surface was lower in the lower Si/Mn ratio sample. The galvannealing reaction was accelerated by decreasing the Si/Mn ratio of the steel. The species and morphology of the Si-containing oxides determined the galvannealing behavior of the Si-added steel. The Si-containing selective surface oxide affected the formation of the initial Fe-Zn intermetallic compounds (IMC) during hot-dipping in molten Zn. The formation of SiO2 was suppressed in the sample with the lower Si/Mn ratio, which resulted in accelerated Fe-Zn IMC formation. On the other hand, solute Si in the steel affected the growth of the Fe-Zn IMC during heating in the galvannealing process. The content of solute Si was assumed to be lower in the lower Si/Mn ratio sample, which resulted in acceleration of Fe-Zn IMC growth.
View full abstractDownload PDF (3035K) Full view HTML -
Naoki Takata, Kunihisa Hayano, Asuka Suzuki, Makoto Kobashi2019 Volume 105 Issue 7 Pages 693-700
Published: 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESS FULL-TEXT HTMLIn order to understand the effect of solute Si (in the steel sheet) on the interfacial reaction between liquid Zn and solid Fe (α-Fe phase) during the hot-dip galvanizing process, a change in the interfacial microstructure between Zn coating and Fe substrate in Fe-Si alloy sheets hot-dipped in Zn melt with dipping time at 460ºC was examined. In pure Fe sheet, the Fe-Zn intermetallic layers form at the interface between solid Fe and liquid Zn at an early stage of dipping and subsequently grow to approximately 60 μm in thickness after 600 s. In Fe-1Si (wt%) alloy, the thickness of Zn coating substantially increases to beyond 500 μm after 600 s and the coarse ζ-FeZn13 phase with several facet planes was often observed in the Zn coating. The thickness of the Fe-1Si alloy sheets continuously decreases till 60 s and then is reduced significantly after 600 s. The thickness loss in the later stage of dipping is more significant in the Fe-Si alloy with higher Si content. These results indicate a significant Fe dissolution into liquid Zn could occur at the later stage of dipping the Fe-1Si alloy in Zn melt, which is distinguished from the interfacial reaction between pure Fe and liquid Zn. The enhanced interfacial reaction would be responsible for the decomposition of the initially formed ζ-FeZn13 phase layer to liquid and FeSi phases, which can be proposed based on thermodynamic calculations of the Fe-Zn-Si ternary system.
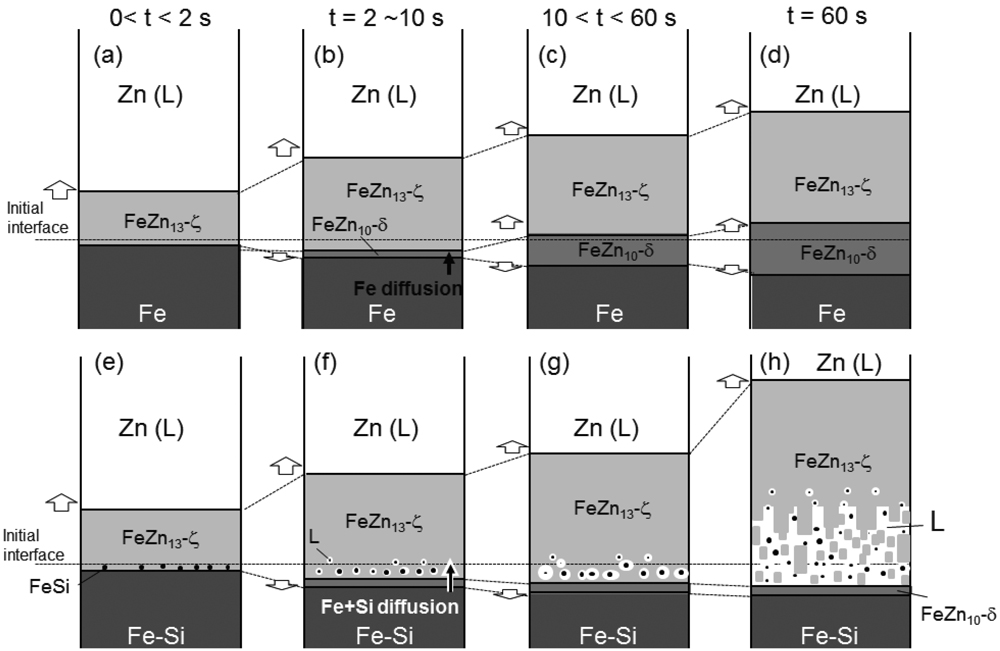 View full abstractDownload PDF (4806K) Full view HTML
View full abstractDownload PDF (4806K) Full view HTML -
Naoki Takata, Kunihisa Hayano, Asuka Suzuki, Makoto Kobashi2019 Volume 105 Issue 7 Pages 701-708
Published: 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESS FULL-TEXT HTMLWe have characterized η-Fe2Al5 phase layers formed on Fe-Si binary alloys (pure Fe, Fe-0.2Si and Fe-1Si (wt%)) hot-dipped in a Zn-0.2Al (wt%) alloy melt at 460ºC for various times ranging from 2 to 3600 s. The Al addition in the Zn melt suppressed the interfacial reaction between liquid Zn and α-Fe phase with Si in solution. At an early stage of dipping (less than 10 s), Fe dissolution into the Zn-0.2Al alloy melt occurs more significantly on the α-Fe phase with higher Si content. The thickness of Fe2Al5 phase layer becomes slightly larger on α-Fe phase with higher Si content. These indicates that solute Si in α-Fe phase could enhance the Fe dissolution providing the driving force for the formation of η phase in liquid Zn alloy, resulting in the enhanced formation of η phase layer. The thicker η phase layer formed on Fe-Si alloy sheets would play a role of diffusion barrier, resulting in the suppression of subsequent interfacial reactions even after 3600 s in dipping.
 View full abstractDownload PDF (4903K) Full view HTML
View full abstractDownload PDF (4903K) Full view HTML -
Sho Katsura, Ryo Sasaki, Noriaki Nakatsuka, Hideyuki Yasuda2019 Volume 105 Issue 7 Pages 709-715
Published: 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESS FULL-TEXT HTMLIn-situ X-ray transmission imaging experiments were performed to the simulated molten zinc baths of CGL in order to clarify the nucleation and growth behavior of Fe2Al5 solid intermetallic compound called “top dross”. Zn alloy samples containing a small amount of Fe and Al were melted in a high vacuum atmosphere and cooled by various rates. The in-situ X-ray transmission observations were performed during the cooling process. The nucleation and growth behavior of Fe2Al5 particles were observed directly. The nucleation behavior is strongly affected by cooing rate, as cooling rate gets faster, the average particle size gets smaller and the number of particles increases. The total volume of crystallized particles is not changed by cooling rate. It is suggested that we have a possibility to control their size or number of dross particles by controlling bath temperature. Their nucleation behavior also suggested that they need a certain amount of Al, Fe supersaturation or supercooling in order to nucleate new dross particles in the bath. The growth speed of the particles in the molten zinc was also analyzed by image analysis. It is suggested that the growth behavior of Fe2Al5 particles is restricted by the diffusion of Fe, Al components. In-situ X-ray imaging method can be a promising technique to understand the nucleation or growth behavior of dross particles generated in CGL baths.
View full abstractDownload PDF (2245K) Full view HTML -
Takeshi Konishi, Junpei Miki, Mina Shibata, Yuu Nemoto, Kohsaku Ushiod ...2019 Volume 105 Issue 7 Pages 716-723
Published: 2019
Released on J-STAGE: June 30, 2019
Advance online publication: November 01, 2018JOURNAL OPEN ACCESS FULL-TEXT HTMLIn a molten zinc bath in a continuous galvanizing line (CGL), top dross particles crystallize as Fe-Al-Zn intermetallic compounds. These particles easily adhere to the steel sheets causing surface defects. Therefore, controlling the top dross particles is a key issue. The present study focused on the structural and mechanical characterizations of top dross particles using an electron probe micro analyzer, X-ray diffraction, electron back scattering diffraction, Vickers hardness measurement and nano-indentation measurement. The following results were obtained: (1) The crystal structure of top dross particles Fe2Al5Znx having Fe: 37~38 wt%, Al: 44~45 wt% and Zn: 18~19 wt% belongs to the orthorhombic system with a lattice constant of a=7.61 Å, b=6.48 Å and c=4.23 Å. The a axis of Fe2Al5Znx becomes shorter, while the b and c axes become longer compared to those of binary Fe2Al5. (2) The top dross particles with the faceted interface were postulated to coarsen by the mechanism of the anisotropic interface energy between the top dross particles and molten Zn as a driving force rather than by the aggregation mechanism. (3) The hardness and the elastic modulus of the top dross particles are the lowest in the [001] direction like Fe2Al5, and are lower than those of Fe2Al5. (4) The fracture toughness of top dross particles is approximately 1.1 MPa·m1/2, which is slightly lower than that of Fe2Al5.
 EBSD (a) image quality map, (b) phase map, and inverse pole figure maps of (c) Fe2Al5Znx phase dross particles and (d) Zn. Fullsize ImageView full abstractDownload PDF (5462K) Full view HTML
EBSD (a) image quality map, (b) phase map, and inverse pole figure maps of (c) Fe2Al5Znx phase dross particles and (d) Zn. Fullsize ImageView full abstractDownload PDF (5462K) Full view HTML -
Aimi Uchiyama, Kohei Kawasaki, Yuji Sutou, Daisuke Ando, Junichi Koike2019 Volume 105 Issue 7 Pages 724-732
Published: 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESS FULL-TEXT HTMLImprovement of the powdering resistance of galvannealed (GA) coatings is a key issue in automotive GA steel sheets. To make the relationship between powdering resistance and microstructure of GA layer, sputtering fabrication process was used to prepare Zn-Fe intermetallic films with various composition and microstructure in this study. Zn-Fe films with 1500 nm in thickness were deposited on an iron substrate by RF magnetron sputtering. Г+Г1 two-phase film (17.2~24.4 at.%Fe) was found to show a severe powdering by 3-point bending test, while Г1 (16.2 at.%Fe) or Г (34.3 at.%Fe) single-phase films showed much better powdering resistance. These results indicate that Г/Г1 interfaces boundaries have a low interfacial strength compared to that of Г/iron or Г1/iron interfaces. To improve the low powdering resistance of the Г+Г1 two-phase film, we investigated the effect of grain size refinement by B addition on the powdering resistance. It was found that the grain size refinement by B addition drastically improves the powdering resistance of Г+Г1 two-phase film. The decrease in the fracture toughness of Г+Г1 two-phase film by grain refinement was suggested to cause the improvement of its powdering resistance.
View full abstractDownload PDF (2423K) Full view HTML -
Kayo Hasegawa, Motoaki Morita, Shinichi Motoda2019 Volume 105 Issue 7 Pages 733-741
Published: 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESS FULL-TEXT HTMLTo understand the fatigue mechanism of hot-dip galvanized steel, the fatigue strength and fracture surface of hot-dip galvanized S45C (carbon steel) specimens were investigated. The galvanized coating layer was composed of δ1-phase, ζ-phase and η-phase, and its thickness was about 100 μm. In low cycle range (104 cycles < Nf <105 cycles), the fatigue strengths of both the carbon steel and the galvanized steel corresponded to the static strength. The fatigue strength of the galvanized steel was lower than that of the carbon steel. As the number of cycles increased, the difference between fatigue strength of the carbon steel and that of the galvanized steel increased. In addition, the morphologies of the fatigue fracture were also different in low cycle range and high cycle range. In the galvanized steel, the morphology of stage II crack on the fracture surface at low cycle range exhibited crescent shape, and multiple crack initiation sites in low cycle range were observed. On the other hand, the morphology at high cycle region (Nf > 105 cycles) exhibited an ellipse shape, and the crack initiation site was single. At both ranges, the crack initiation sites were in the coating layer. The mechanical properties of the microstructure in coating layer affect on the fatigue strength. When η-phase was removed from the galvanized coating layer, the fatigue strength increased only in high cycle range. Therefore, δ1-phase and/or ζ-phase cause the fatigue strength to decrease in low cycle range, and η-phase causes it in high cycle range.
View full abstractDownload PDF (4016K) Full view HTML -
Masayuki Yamamoto, Keiji Murayama, Hongmei Li, Naoki Takata2019 Volume 105 Issue 7 Pages 742-751
Published: 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESS FULL-TEXT HTMLWe examined the effect of boron (B) addition (up to 10 ppm) on the liquid zinc (Zn) embrittlement of 490 MPa grade steels (SN490B) containing a trace amount (approximately 70 ppm) of nitrogen (N) via notched-bar tensile (NBT) tests. The studied steels were heat-treated in order to reproduce the microstructure (martensite structure) of the heat -affected zones in the building structural steels. The NBT tests indicate a slight effect of B addition on the liquid Zn embrittlement of the studied steels containing trace N; this is different from the results of previous studies on the liquid Zn embrittlement of N-free steels. However, the addition of titanium (Ti) into the studied steels induces remarkable liquid Zn embrittlement. The NBT-tested specimens of the Ti-added steels exhibit a brittle fracture surface, indicating intergranular fracture induced by liquid Zn. Its associated cracks preferentially propagate along prior austenitic boundaries, and Zn is enriched at these crack tips. Minute chemical analysis reveals a significant segregation of B at prior austenitic boundaries in the Ti-added steels. These results indicate that the segregated B would facilitate crack propagation along the prior austenitic boundaries induced by liquid Zn, resulting in enhanced liquid Zn embrittlement. The effects of N and Ti on B segregation and the related liquid Zn embrittlement are discussed.
View full abstractDownload PDF (12669K) Full view HTML -
Yuki Suzuki, Shinichi Yamaguchi, Masamitsu Matsumoto, Izumi Muto2019 Volume 105 Issue 7 Pages 752-758
Published: 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESS FULL-TEXT HTMLZinc and zinc alloy coated steel is widely utilized for home appliance, construction, automobile, and so on for its high corrosion resistance. In this work, corrosion behavior at cut edges of Zn-11%Al-3%Mg-0.2%Si alloy coated steel sheets (SD) was investigated with cyclic wet-dry corrosion test. The result showed that SD have the superior anti-corrosive property to zinc coated steel sheets (GI) at early period of corrosion. GI produced red rust, whereas SD produced no red rust. After the cyclic wet-dry corrosion test, zinc-containing white rust deposited on steel substrate. In the case of SD, magnesium reached the center of cut edge and larger area on the steel was covered with white rust. With polarization measurements of steel substrate on which white rust deposited, it was clarified that white rust of SD reduced both anodic and cathodic current density of steel substrate more largely than that of GI. In the case of SD, galvanic current between steel substrate with white rust and coating layer was small compared with GI. It was suggested this anti-corrosive property of SD is due to magnesium-containing white rust.
View full abstractDownload PDF (3487K) Full view HTML -
Jun Maki2019 Volume 105 Issue 7 Pages 759-766
Published: 2019
Released on J-STAGE: June 30, 2019
JOURNAL OPEN ACCESS FULL-TEXT HTMLAluminized steel sheets are very resistant to corrosion in the outdoor exposure environment. We evaluated the corrosion behavior of aluminized steel sheets with a Type1 coating containing around 10%Si but also a Type2 coating not containing Si in a 50-year outdoor exposure test. Both specimens had strong perforation resistance, but those with Type2 coating had superior perforation resistance. The Type2 aluminized steel sheets had two sublayers composed of Fe2Al5 and FeAl2 as the intermediate layer between the aluminized layer and the steel substrate. The FeAl2 phase has less noble potential than steel substrate and the Fe2Al5 phase in an artificial rain water environment. As a result, this layer provided sacrificial corrosion protection for the steel substrate. That was why the specimens with the Type2 coating had better perforation resistance than those with Type1 coating.
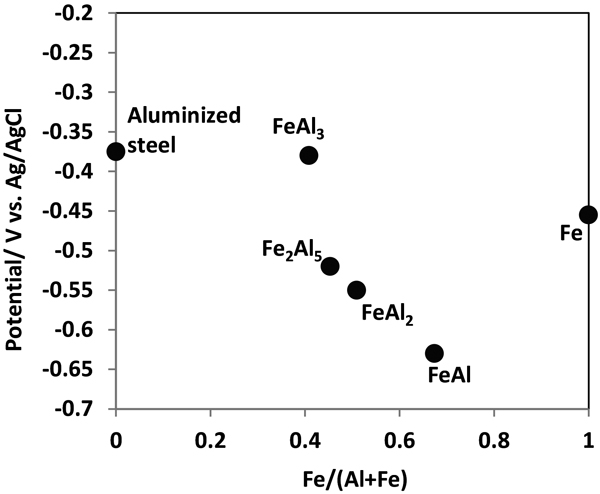 Corrosion potential of intermetallic compound, steel substrate and aluminized steel sheets in an artificial rain water. Fullsize ImageView full abstractDownload PDF (15393K) Full view HTML
Corrosion potential of intermetallic compound, steel substrate and aluminized steel sheets in an artificial rain water. Fullsize ImageView full abstractDownload PDF (15393K) Full view HTML
- |<
- <
- 1
- >
- >|