2015 Volume 55 Issue 7 Pages 1347-1352
2015 Volume 55 Issue 7 Pages 1347-1352
Waste copper slag is a refractory material with high iron content, but it is difficult to recovery iron minerals from the slag because the iron mainly occurs in fayalite. A new technology of coal-based direct reduction-magnetic separation process was developed to recover iron from the slag assaying 39.85% Fetotal and 0.33% Cu. The results show that the final iron concentrate, assaying 90.68% Fetotal, 94.01% metallization degree, 0.66% Cu and 0.058% S with overall iron and copper recoveries of 90.49% and 79.53%, respectively, was manufactured under the optimized conditions as follows: balling the mixture of copper slag with 20% compound additive at 0.3 of basicity, preheating the green pellets at 1000°C for 9 min, then reducing the preheated pellets at 1200°C for 70 min with coal to dried pellets mass ratio of 2, grinding the reduced pellets up to 95% passing 0.074 mm, and magnetically separating the ground product in Davi Tube at 0.08 T magnetic field intensity. The observation of the reduced pellets microstructure shows that the additive plays a role of nucleating agent, which enhances metallic iron grains migrating and coarsening during reduction process. The process is probably one of efficient ways to recover iron from copper slag to produce directly reduced iron (DRI), which can be used as the burden to produce weathering resistant steel by electric arc furnace to replace sponge iron or scrap steel. The process reduces the secondary environmental pollution of copper slag and has been applied well in Tongling Nonferrous Metals Group Holding Co., Ltd in China.
Copper slag is a waste during pyrometallurgical production of copper from copper sulfide concentrates, about 2.2 tons of which being generated per ton of metal copper production. Dumping or disposal of such huge quantities of slag causes not only secondary environmental pollution due to the high content of Cu, Pb, Zn and S in copper slag, but also wastes resources because of about 40% iron and some Cu, Pb, Zn occurring in copper slag.1)
Extensive researches were conducted to utilize copper slag. Various methods of recovering copper from copper slag including pyrometallurgical, hydrometallurgical, and combined pyro and hydrometallurgical processes were briefly reviewed by Gorai et al.2) The recovery of copper by floatation from copper slag is the most effective and successful route.3,4,5,6) However, many beneficiation processes to separate the copper from slag are not economical because of the low grade of copper. In the meantime, the slag can be directly used in other areas, such as cement, tiles, binder and so on.2) Using of copper slag as a Portland cement replacement was reported by several researchers.7,8,9) It affects the strength and toughness of the mixture. Recover of value metals by ammonium chloride treatment was proposed by Nadirov et al.10) Few literatures about recovering iron from copper slag were reported, because it is difficult to extract iron from the slag by traditional processes, where iron mainly exists in fayalite (Fe2SiO4). Cheng et al. developed a method to directly upgrade iron from the slag.11) However, no results of iron grade and recovery were reported. Kim et al. carried out a research on reduction-magnetic separation process, where reduction at 1250°C for 1.5 h and then followed by dry magnetic separation to recover iron from the copper slag of Korea Zinc Co., Ltd., but the grade of product is low and SiO2 content is as high as 12.7%.12) Li et al. extracted the iron by deep reduction at 1300°C for 3 h and magnetic beneficiation, producing iron powder containing 96.21% Fetotal.13)
Direct reduction is one of effective technologies to process the secondary resources containing ferrous. Due to the lack of natural gas, coal-based direct reduction is the preferentially considered technique in China. Coal acts as not only exothermal agent but also reductant, and deoxidation reactions are completed in rotary kiln or tunnel kiln.14,15) Therefore, an innovative technology of recovering iron from copper slag by a coal-based direct reduction and magnetic separation process was developed in this paper.
The chemical compositions of copper slag used in this study are given in Table 1. The total iron content of copper slag is 39.85%, and the content of SiO2, Cu, Pb and Zn are 30.81%, 0.33%, 0.22% and 2.81%, respectively. The minerals of copper slag determined by X-ray diffraction (XRD) are mainly fayalite and magnetite, as shown in Fig. 1. Figure 2 presents representative SEM-EDS micrographs of copper slag sample, which indicates that fayalite and magnetite are the main minerals in slag, and they are closely combined with copper matte and glassy minerals. Sphalerite is found jointed with copper matte. The size of copper slag is 84.28% and 96.74% passing 0.044 mm and 0.074 mm, respectively.
| Element | Fetotal | FeO | SiO2 | CaO | MgO | Al2O3 | Cu | Pb | Zn | S | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 39.85 | 43.68 | 30.81 | 2.00 | 1.28 | 2.83 | 0.33 | 0.22 | 2.81 | 0.18 | 0.19 |

X-ray diffraction pattern of copper slag.

Representative SEM-EDS micrographs of copper slag sample (C – Copper matte, Fa – Fayalite, M – Magnetite, Sp – Sphalerite, G – Glassy materials).
Limestone containing 60.38% of CaO and 37.47% of loss of ignition (LOI) was used to adjust the basicity of blends.
The compound additive containing 16.38% of hematite, 41.45% of magnetite and 35.18% of LOI was produced by Central South University, in a form of powder with over 90% passing 0.074 mm.
The soft coal was used as reductant agent, with fixed carbon of 52.12% on air dry basis (FCad), volatile matter of 30.41% on dry ash free (Vdaf), ash of 4.49% on air dry basis (Aad), 0.58% of S and melting temperature of 1376°C. The size of soft coal is 100% passing 3 mm.
2.2. Experimental MethodsThe experimental flow sheet includes procedures as follows: mixing the copper slag with additive, pelletizing of mixture, preheating of dried pellets, reduction roasting of preheated pellets, grinding the reduced pellets, low intensity magnetic separation of the ground product to manufacture DRI.
Mixtures were prepared by mixing copper slag, limestone and some compound additive at a certain ratio relative to the mass of copper slag. Then pellets were prepared by balling the mixtures in a disc pelletizer of 0.8 m in diameter and 0.2 m rim depth, rotating at 38 rpm and being inclined at 47° to the horizontal. The green balls were transferred into the drying oven to dry at 105°C for 2 h until the weight unchanged.
The dried pellets were put into the corundum porcelain and were preheated in the horizontal tube, where the preheating temperature from 900 to 1100°C and duration from 6 to 15 min were investigated to get the suitable preheating conditions, and the best compressive strength of preheated pellets was 1270 N/pellet by preheating at 1000°C for 9 min.
The coal-based direct reduction was done as follows: the preheated pellets were covered with proper mass of coal in the stainless steel pot. The steel pot was put into the muffle furnace (model: KSY-12-18) while the reducing temperature was elevated to the target value. When the reduction time was ended, the hot steel pot was taken out and covered by pulverized coal to cool down quickly in the air to prevent reduced pellets from being re-oxidized.
The magnetic separation was performed in the Davis Tube (model: XCGS-73) after 20 g of reduced pellets being ground in the cone ball mill (model: XMQ240×90). The DRI was obtained after magnetic separation. The grinding fineness of reduced pellets was about 95% passing 0.074 mm and the magnetic field intensity was 0.08 T.
The chemical compositions of copper slag and iron concentrate were measured using chemical analysis. The crystalline phase composition of the material was investigated using X-ray diffractometer (XRD, RIGAKU, D/Max-2500). Proximate analysis of coal and determination of fusibility of coal ash were followed by GB/T212-2008 and GB/T219-2008, respectively. Microstructures of reduced briquettes were analyzed by scanning electron microscope (SEM, FEI Quanta-200), and the compositional analyses were carried out using an energy dispersion system (EDAX-TSL, Ametek) within the SEM.
Binary basicity was used in the tests, which is the ratio of CaO/SiO2. The effect of basicity on the beneficiation of iron concentrate is presented in Fig. 3. The iron grade decreased from 71.02% to 63.52% and the iron recovery increased from 55.05% to 74.78% when basicity was elevated from 0.065 (natural basicity) to 0.9. By adjusting the basicity, more iron was transformed to metallic iron, leading to higher recovery. But iron grains were still small (shown in Sec. 3.6) and combined with impurities, issuing in lower iron grade of concentrate.

Effect of basicity on the beneficiation of iron (Reducing at 1100°C for 70 min with coal to dried pellets mass ratio of 2).
The thermodynamics calculation results of fayalite reduction process by CO and adding with alkaline oxide are shown in Fig. 4. It indicates that fayalite is difficult to be reduced only by CO [Eq. (1)], but easier by added alkaline oxide [Eq. (2)]. CaO can improve the fayalite reduction by chemically combining with SiO2 to form wollastonite (CaO·SiO2).11) More CaO will help to form dicalcium silicate (2CaO·SiO2) by solid-phase reaction, which contributes to the separation of slag and iron by natural pulverization of dicalcium silicate during cooling.11,16)
| (1) |
| (2) |

Change of Gibbs free energy in direct reduction process on fayalite.
By consideration of iron grade and recovery, the suitable basicity was suggested at 0.3, where the iron grade and recovery are 69.48% and 69.86%, respectively. However, it can’t get high quality of DRI only by adjusting the basicity, additive should be developed to enhance the reduction and grain growth of iron.
3.2. Dosage of AdditiveSodium salts were developed by many researchers to promote the reduction of iron from low grade and complex iron ores, such as high-aluminum limonite ore,17) high-phosphorus oolitic hematite ore18) and siderite ore,19) and good results were achieved. However, sodium salts may cause the degradation of refractory materials,20) so a compound additive containing low impurities, mainly in iron oxides and volatile matter content, was developed to improve reduction on low grade hematite pellets.21,22) And the additive was used in the tests of extracting iron from copper slag. Figure 5 shows the effect of additive dosage on the upgrading of iron concentrate. The compound additive can enhance upgrading the iron, leading to both higher iron grade and recovery. The iron grade was raised from 69.48% to 78.18% and iron recovery was elevated from 69.74% to 89.37% when dosage of the compound additive was augmented from 0% to 20%. The indexes of iron concentrate kept unchanged when dosage of additive was beyond of 20%. Therefore, the optimum dosage of additive was advised at 20%.

Effect of dosage of additive on the beneficiation of iron (0.3 of basicity, reducing at 1100°C for 70 min and at coal to dried pellets mass ratio of 2).
Although additive can reinforce the indexes of iron concentrate in a certain extent, but rational temperature and duration should be optimized to extract better quality of iron powder.
3.3. Reduction TemperatureThe effect of reduction temperature on the upgrading of iron is illustrated in Fig. 6. The iron grade raised from 78.18% to 91.16% when the reduction temperature increased from 1100°C to 1250°C, and the iron recovery was over 90%. Temperature is an important factor in direct reduction, and increasing temperature can obviously promote reducing reactions. The higher the reduction temperature, the higher iron grade obtained.

Effect of reduction temperature on the beneficiation of iron (0.3 of basicity, blending with 20% of compound additive, reducing for 70 min with coal to dried pellets mass ratio of 2).
The effect of reduction time on the upgrading of iron is presented in Fig. 7. When reduction time was extended from 30 to 70 min, the iron grade increased from 74.81% to 90.68% and iron recovery enhanced from 88.56% to 90.49%, and then kept steady when time was beyond of 90 min. Metallic iron is strongly magnetic mineral, which can be easily to recover by low-intensity magnetic separation process. So that high recoveries around 90% were achieved and didn’t change much with an increase of the reduction time. Therefore, reducing for 70 min at 1200°C is recommended.

Effect of reduction time on the beneficiation of iron (0.3 of basicity, blending with 20% of compound additive, reducing at 1200°C with coal to dried pellets mass ratio of 2).
Table 2 summarizes the effect of mass ratio of coal to dried pellets on the concentration of iron. When coal to dried pellets mass ratio was elevated from 1 to 2, the iron grade and recovery increased from 88.64% to 90.68% and 88.70% to 90.49%, respectively. The iron grade and recovery kept steady when the ratio was beyond 2. The ratio is higher than industrial application with a degree of 0.5–0.7, which is similar with the research developed by Zhu et al.23)
| Mass ratio of coal to dried pellets | Iron grade (mass, %) | Iron recovery (%) |
|---|---|---|
| 1:1 | 88.64 | 88.70 |
| 2:1 | 90.68 | 90.49 |
| 3:1 | 90.12 | 91.19 |
In summary, the optimum conditions from above tests recommended were as followings: preparing the green balls by blending with 20% of compound additive and 0.3 basicity, preheating dried balls at 1000°C for 9 min, reducing the preheated pellets at 1200°C for 70 min with coal to dried pellets mass ratio of 2, and magnetic separating the ground reduced briquettes with a size up to 95% passing 0.074 mm at 0.08 T magnetic field intensity.
The chemical compositions of final iron powder are shown in Table 3. The DRI, assaying 90.68% Fetotal, 94.01% metallization degree, 0.66% Cu, 0.021% Pb, 0.041% Zn and 0.058% S with overall iron and copper recoveries of 90.49% and 79.53%, respectively, was manufactured. The final product can be used as the burden along with scrap steel for making weathering resistant steel by electric arc furnace. The process has been applied well in Tongling Nonferrous Metals Group Holding Co., Ltd in China.
| Element | Fetotal | Femetal | SiO2 | CaO | Al2O3 | Cu | Pb | Zn | S | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 90.68 | 85.25 | 3.90 | 0.97 | 0.40 | 0.66 | 0.021 | 0.0041 | 0.058 | 0.081 |
Figure 8 shows the microstructures of reduced pellets and chemical compositions without additive under SEM and EDS. Metallic iron grains are pure and embrace some of metal Cu (Phase 1 in Fig. 8). The size of metallic ferrous grains is smaller than 20 μm, indicating that metallic ferrous grain is difficult to be liberated from gangue minerals and effective upgraded by magnetic separation. Meanwhile, some wustite inclusions present in metallic ferrous grains (Phase 2 in Fig. 8), which affects the iron grade of DRI. According to EDS analysis (Phase 3 in Fig. 8), the main gangue mineral with an atom ratio [Si]/[Fe]=1.30 is mainly consist of ferrosilite (FeO·SiO2, with an atom ratio [Si]/[Fe]=1), leading to low recovery of iron.

SEM-EDS results of reduced pellets without additive (0.3 of basicity, reducing at 1200°C for 70 min at coal to dried pellets mass ratio of 2).
By contrast, the compound additive can significantly promote the growth of metallic ferrous grains to around 50 μm (Fig. 9), inferring that easy liberation and high-grade iron concentrate will be obtained by magnetic separation. The compound additive contains iron minerals of hematite and magnetite, which play a role of nucleating agent to reduce the nucleating barrier, leading to improve the aggregation and growth of metallic iron grains.21) According to EDS analysis (Phase 2 in Fig. 9), the main gangue minerals with an atom ratio [Si]/([Fe]+[Ca])=1.16 are mainly consist of wollastonite (CaO·SiO2, with an atom ratio [Si]/[Ca]=1) and ferrosilite (FeO·SiO2), but the content of ferrosilite is lower than Phase 3 in Fig. 8. Minerals of Phase 3 in Fig. 9 are similar with Phase 2.
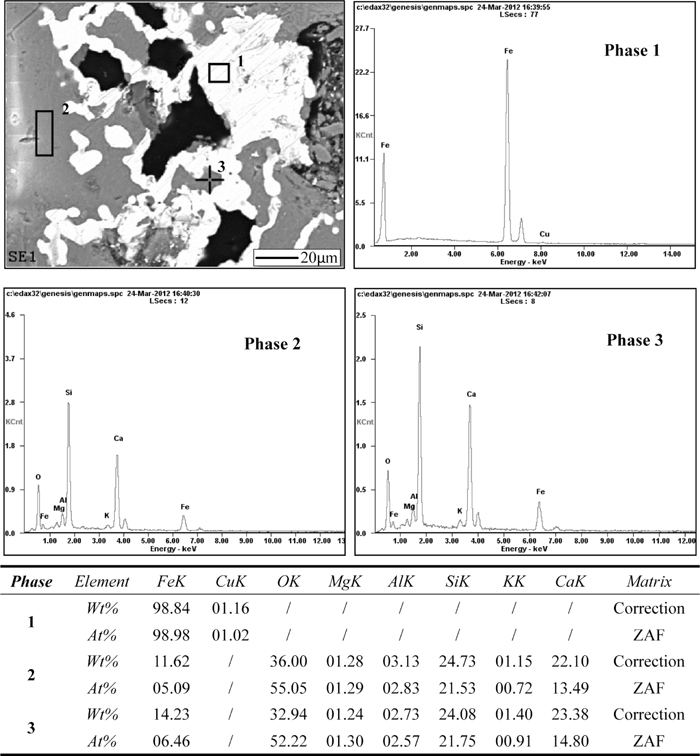
SEM-EDS results of reduced pellets with 20% compound additive (0.3 of basicity, reducing at 1200°C for 70 min at coal to dried pellets mass ratio of 2).
(1) The copper slag, assaying 39.85% Fetotal, 30.81% SiO2, 0.33% Cu, 0.22% Pb, 2.81% Zn and 0.18% S, was used as the raw materials to produce metal ferrous powder. Iron minerals mainly exist in the form of fayalite and magnetite, which are closely combined with copper matte and glassy minerals, issuing in difficult to extract iron from the slag.
(2) The final DRI, assaying 90.68% Fetotal, 94.01% metallization degree, 0.66% Cu, 0.021% Pb, 0.041% Zn and 0.058% S with overall iron and copper recoveries of 90.49% and 79.53%, respectively, was manufactured under the following conditions: pelletizing the mixture of copper slag with 20% compound additive at 0.3 of basicity, preheating the pellets at 1000°C for 9 min, then reducing the preheated pellets at 1200°C for 70 min with coal to dried pellets mass ratio of 2, grinding the reduced pellets up to 95% passing 0.074 mm and magnetic separation in Davi Tube at 0.08 T magnetic field intensity. The process reduces the secondary environmental pollution of copper slag and has been applied well in Tongling Nonferrous Metals Group Holding Co., Ltd in China.
(3) The compound additive plays a role of nucleating agent during direct reduction, which can significantly enhance the reduction of fayalite, improve metallic ferrous grains migrated and agglomerated, effectively promote the iron grains liberation in grinding, upgrading the iron degree and iron recovery in magnetic separation. Further work will be taken to verify the reduction behavior of compound additive during reduction process.
This work was financially supported by the National Key Technology R&D Program of China (NO. 2013BAB03B04).