2023 Volume 5 Issue 1 Pages 5-12
2023 Volume 5 Issue 1 Pages 5-12
BACKGROUND
Epidemiological data are essential for developing strategies against the current coronavirus disease 2019 (COVID-19) pandemic. Data on COVID-19 epidemiology in Japan are limited owing to a focus on specific regions and patient groups, particularly in the early phase of the pandemic.
METHODS
We investigated COVID-19 epidemiology in Japan in 2020 using a large nationwide multihospital database containing insurance claim records and medical records. Inclusion criteria were inpatient and outpatient referrals for COVID-19 in 2020. We analyzed demographic data, comorbidities, drug use, severe COVID-19 risk, and clinical course of hospitalized patients (including death).
RESULTS
We identified 11,868 COVID-19 cases from 56 institutions: 6,440 outpatients and 5,428 inpatients. Of the patients, 53.2% had comorbid conditions, the most common of which was tumor (22.1%), and 56.4% were classed as having a high risk of COVID-19. Pharmacological management patterns were generally consistent between the first and second half of 2020, except for glucocorticoid use. The use of unauthorized medications (hydroxychloroquine, ivermectin, and favipiravir) was infrequent. For hospitalized patients, the median length of stay was 10 days, and 2.4% of patients were admitted to intensive care units. Post-COVID-19 all-cause mortality, all-cause 30-day mortality, and in-hospital deaths were recorded for 7.9%, 5.4%, and 4.6% of patients, respectively. Patients with high-risk conditions had a lower survival probability.
CONCLUSIONS
This descriptive study of COVID-19 in 2020 identified differences in care across outpatient and inpatient settings and changes in care delivery as the pandemic progressed. These findings could inform strategies for future infectious disease pandemics.
The global burden of the coronavirus disease 2019 (COVID-19) pandemic has been considerable. By the end of 2021, more than 300 million confirmed cases and over 5 million related deaths had been cumulatively reported, and the daily number of deaths has continued to rise substantially [1]. It is possible that this burden has been underestimated owing to underreporting from developing countries [2, 3]. In Japan, nearly 1.9 million COVID-19 cases and 18,000 attributable deaths have been reported as of December 2021 [4].
COVID-19 epidemiological factors, such as pandemic size, management, prognosis, and resource utilization [5–9], have evolved over time and may vary across countries [10]. Country-specific data are invaluable in 1) understanding how people respond to the pandemic; 2) planning a strategy to minimize the pandemic disease burden (e.g., resource allocation or financial incentives); and; 3) preparing for the next infectious disease pandemic. However, data on COVID-19 epidemiology in Japan are limited. For example, studies have been focused on hospitalized patients or populations with specific risk factors or have relied on regional registry data [11–15].
In this report, we describe hospital-based COVID-19 epidemiology in Japan in 2020 using a nationwide multihospital database. The aim was to expand knowledge of the broader patient population by examining both outpatient and inpatient care.
This study was conducted with the approval of the Japan Medical Association Ethical Review Board (R2-5). Because the data were anonymized, the requirement to obtain patient informed consent was waived.
DATA SOURCEWe used the RWD database [16, 17], which is maintained by the Health, Clinic, and Education Information Evaluation Institute (Kyoto, Japan) and managed by Real World Data Co., Ltd (Kyoto, Japan). The database contains the records of approximately 20 million patients from around 160 medical institutions, as of 2020. Contributing hospitals are located across Japan, although they tend to cluster in western regions. The database comprises demographic data, diagnoses, prescriptions, and procedures from both outpatient and inpatient services. Prescription data from electronic medical records are drawn from physician medication orders; this enables the extraction of information on any drug prescriptions, including those not covered by insurance (e.g., drugs used in clinical trials). Other data were obtained from insurance claim records and/or electronic medical records, such as physician orders.
The data were automatically extracted from the electronic medical records of each medical institution. Patient records are managed by the allocation of unique identifiers to each individual; these identifiers are used consistently within each institution.
In addition, two hospitals outside the RWD database offered data on patients with COVID-19 in response to a request from the Japan Medical Association. These datasets were combined with the RWD dataset and analyzed.
PATIENT INCLUSION CRITERIAPatients with COVID-19 were identified using the International Statistical Classification of Diseases and Related Health Problems, 10th revision, code U07, which is a specific code for COVID-19. Patients assigned a U07 code who had an additional “suspicious” supplemental disease code were excluded from the analysis. The study period was from January 1, 2020, to December 31, 2020. Patients who did and did not require hospitalization were included. If a patient visited a medical institution as an outpatient but was subsequently hospitalized during the same disease course, they were defined as an inpatient. If a patient had multiple episodes of COVID-19 infections, only the first episode was analyzed. There were no exclusion criteria. To ensure that all patients with COVID-19 were enrolled, we did not use a data lookback period [18].
COMORBIDITY AND RISK OF SEVERE DISEASEComorbid conditions for each patient were summarized. The disease codes used to classify these diseases are shown in Table 1. If the codes were assigned before or on the date of COVID-19 diagnosis, we regarded the conditions as comorbidities.
| Disease/condition | codes |
|---|---|
| COVID-19 | U07 |
| Diabetes | E10-14 |
| Tumors1 | C00-97, D00-48 |
| malignant tumors | C00-97 |
| Cardiovascular disease | I20-25, I50 |
| Stroke | I60-69 |
| Chronic clung disease2 | J40-47 |
| Hypertension | I10-15 |
| Liver disease | B15-19, C22, K70-77 |
| Renal disease | N17-19 |
| Obesity | E65-68 |
1: Both benign and malignant tumors are involved.
2: Including asthma and chronic obstructive pulmonary disease.
ICD-10: International Statistical Classification of Diseases and Related Health Problems, 10th Revision, COVID-19: coronavirus disease 2019.
In this study, we defined a high risk of severe COVID-19 as one of the following: aged ≥65 years, chronic kidney disease, type 1 or type 2 diabetes, chronic lung disease, cardiovascular disease (including cerebrovascular disease), and malignant tumor [19–23]. These conditions were identified using medical records, claims records, or discharge abstracts (the latter source was only applicable to inpatients admitted to hospitals with a payment scheme based on the Diagnosis Procedure Combination system) [24].
PHARMACOLOGICAL MANAGEMENTFor the included patients, we reviewed prescription data, including systemic glucocorticoids (dexamethasone and other corticosteroids), ciclesonide, remdesivir, hydroxychloroquine, ivermectin, favipiravir, azithromycin, and statins, which were referred to as repurposed drugs used for anti-inflammatory or antiviral effects [25]. We additionally examined prescription records for anticoagulants and antiplatelets because some studies have shown that these drugs can modify the course of COVID-19 [26]. For all drug classes, both prevalent and naïve users were counted. Additionally, inpatient medication use was summarized both for outpatient and inpatient settings. The drugs listed above were not available as over-the-counter medications in Japan.
The repurposed drugs were tested in clinical trials or used without authorization in some settings. Remdesivir was authorized as a therapeutic drug in May 2020 [27]. However, it was not covered by insurance and was provided free of charge by the Japanese government in 2020. Although the information on drug use was extracted from physician order records, there are some institutional administrative procedures where uninsured drugs are not recorded; thus, the use of uninsured drugs (e.g., remdesivir) may be underestimated.
CARE AND OUTCOME OF HOSPITALIZED PATIENTSFor hospitalized patients, we also examined oxygen use, the need for respiratory support, the use of extracorporeal membrane oxygenation, and intensive care unit admission. We also investigated deaths (all-cause mortality after the first COVID-19 diagnosis [index date], all-cause 30-day mortality after the index date, and in-hospital deaths) and length of hospital stay as outcome measures.
STATISTICAL ANALYSISWe used descriptive statistics to summarize patient characteristics and receipt of treatment. Data throughout the fiscal year of 2020 and data during the three epidemic waves in 2020 (i.e., January 1 to June 30, July 1 to October 31, and November 1 to December 31 [28]) are reported separately. Kaplan-Meier curves were plotted to show the survival curves for hospitalized patients, and the log-rank test was conducted to compare survival probability between groups. Follow-up was censored at the last visit to each hospital, death, or December 31, 2020, whichever occurred first. We did not perform adjustments using multivariate analysis because the availability of pharmacological and non-pharmacological treatment options differed even within 2020, and COVID-19 care expertise likely increased over time. Additionally, as mentioned above, the information about medication use may have been incomplete (e.g., medication use before study enrollment because we did not include a data lookback period). Instead of conducting multivariate analysis, data categorized according to 6-month intervals or selected subgroups (e.g., age) were used for some analyses.
All statistical analyses were conducted using the R statistical environment, version 3.63 (https://cran.r-project.org/). A p-value <0.05 indicated statistical significance.
We identified a total of 11,868 cases: 6,440 outpatients and 5,428 inpatients (Table 2). Overall, the median age was 54 years, and 51.8% (6,151/11,868) of patients were men. A total of 84.0% (9,964/11,868) of medical encounters occurred in the second half of 2020, which coincided with the pattern of COVID-19 infections in Japan. The reports were derived from 56 medical institutions; each institution provided care for a median of 47.5 patients (range: 1 to 2,389). Approximately 98% of patients (11,680/11,868) sought care at acute care hospitals.
| Variable | All (n = 11,868) | Outpatient (n = 6,440) | Inpatient (n = 5,428) |
|---|---|---|---|
| Age (yrs)1 | 54 (32, 74) | 44 (28, 66) | 67 (42, 80) |
| Sex (male) | 6,151 (51.8%) | 3,268 (50.7%) | 2,883 (53.1%) |
| Comorbidity | |||
| diabetes | 2,155 (18.2%) | 678 (10.5%) | 1,477 (27.2%) |
| tumors | 2,624 (22.1%) | 1,198 (18.6%) | 1,426 (26.3%) |
| malignancy | 1,860 (15.7%) | 754 (11.7%) | 1,106 (20.4%) |
| CVD | 1,643 (13.8%) | 535 (8.3%) | 1,108 (20.4%) |
| stroke | 1,283 (10.8%) | 437 (6.8%) | 846 (15.6%) |
| CLD | 1,384 (11.7%) | 718 (11.1%) | 666 (12.3%) |
| hypertension | 2,325 (19.6%) | 751 (11.7%) | 1,574 (29.0%) |
| liver disease | 1,169 (9.9%) | 552 (8.6%) | 617 (11.4%) |
| renal disease | 550 (4.6%) | 181 (2.8%) | 369 (6.8%) |
| obesity | 57 (0.5%) | 23 (0.4%) | 34 (0.6%) |
| smoke | Current or Ex: 888 (7.5%) Never: 1,696 (14.3%) NA: 9,284 (78.2%) |
NA: 6,440 | Current or Ex: 888 (16.4%) Never: 1,696 (31.2%) NA: 2,844 (52.4%) |
1: median and interquartile range are reported.
CVD: cardiovascular disease, CLD: chronic lung disease, NA: not available.
Of the 11,868 cases, 6,318 (53.2%) had at least one co-existing disease of those listed in Table 1, of which tumors were the most common (22.1%). High-risk patients accounted for 56.4% of all 11,868 cases: 43.5% of outpatients (2,799/6,440) and 71.7% of inpatients (3,890/5,428), respectively.
In each epidemic wave in 2020, there were 1,964 cases (1,191 outpatients and 773 inpatients), 5,489 cases (2,960 outpatients and 2,529 inpatients), and 4,415 cases (2,289 outpatients and 2,126 inpatients) reported in the first, second, and third waves, respectively. The characteristics of patients in each period are provided in Supplemental Table 1. Patients in the third wave were 7–10 years older (median) than those in earlier waves. Moreover, patients with comorbidities were observed more often during the third wave than during earlier waves.
PHARMACOLOGICAL MANAGEMENTThe pharmacological management that patients received is shown in Table 3. A total of 2,728 (23.0%) patients received any of these therapies, and 1,158 (9.8%) received a combination of these drugs.
| Drug | All (n = 11,868) (1,974, 9,904)1 |
Outpatient (n = 6,440) (1,191, 5,249)1 |
Inpatient (n = 5,428) (773, 4,655)1 |
|---|---|---|---|
| Glucocorticoids | 1,019 (8.6%) (125 [6.4%], 894 [9.0%]) |
137 (2.1%) (25 [2.1%], 112 [2.1%]) |
882 (16.3%) (100 [12.9%], 782 [16.8%]) |
| dexamethasone | 479 (4.0%) (23 [1.2%], 456 [4.6%]) |
62 (2.1%) (8 [0.7%], 54 [1.0%]) |
317 (7.7%) (15 [1.9%], 402 [8.6%]) |
| Ciclesonide | 265 (2.2%) (44 [2.2%], 221 [2.2%]) |
15 (0.2%) (2 [0.2%], 13 [0.3%]) |
250 (4.6%) (42 [5.4%], 208 [4.5%]) |
| Remdesivir | 98 (0.8%) (0 [0%], 98 [1.0%]) |
2 (0.03%) (0 [0%], 2 [0.04%]) |
96 (1.8%) (0 [0%]), 96 [2.1%]) |
| Hydroxychloroquine | 15 (0.1%) (12 [0.6%], 3 [0.03%]) |
2 (0.03%) (1 [0.08%], 1 [0.02%]) |
13 (0.2%) (11 [1.4%], 2 [0.04%]) |
| Ivermectin | 2 (0.02%) (0 [0%], 2 [0.02%]) |
1 (0.02%) (0 [0%], 1 [0.02%]) |
1 (0.02%) (0 [0%], 1 [0.02%]) |
| Favipiravir | 259 (2.2%) (43 [2.2%], 216 [2.2%]) |
2 (0.03%) (0 [0%], 2 [0.04%]) |
257 (4.7%) (43 (5.6%), 214 [4.6%]) |
| Azithromycin | 234 (2.0%) (91 [4.6%], 143 [1.4%]) |
91 (1.4%) (47 [4.0%], 44 [0.8%]) |
143 (2.6%) (44 [5.7%], 99 [2.13%]) |
| Statins | 780 (6.6%) (114 [5.8%], 666 [6.7%]) |
96 (1.5%) (25 [2.1%], 71 [1.4%)) |
684 (12.6%) (89 [11.5%], 595 [12.8%]) |
| Anticoagulants/antiplatelets | 1,446 (12.2%) (217 [11.1%], 1,229 [12.4%]) |
141 (2.2%) (36 [3.0%], 105 [2.0%]) |
1,305 (24.0%) (181 [23.4%], 1,124 [24.2%]) |
1: In parentheses, the medication usage in the first and second half of 2020 are respectively shown.
Anticoagulants and systemic corticosteroids, including dexamethasone, were used to treat up to 24.0% and 16.3% of inpatients, respectively, whereas the repurposed use of remdesivir, hydroxychloroquine, ivermectin, and favipiravir were less frequently recorded in both outpatient and inpatient settings. Overall, the patterns of pharmacological management were stable across the first and second halves of 2020; however, there was an increase in dexamethasone use in hospitalized patients (Table 3). Prescription patterns of the three epidemic waves are presented in Supplemental Table 2.
CARE AND OUTCOME OF HOSPITALIZED PATIENTSOf 5,428 hospitalized patients, 16.5%, 3.8%, and 0.4% received oxygen (alone), mechanical ventilation, and extracorporeal membranous oxygenation therapy, respectively. Intensive care unit admission was recorded in 128 cases (2.4%). Of the patients who required respiratory support or intensive care, 122 (50.4%) received pharmacological therapeutics to treat COVID-19.
The median length of hospital stay was 10 days, with an interquartile range of 5 to 17 days. All-cause mortality post-COVID-19, all-cause 30-day mortality, and in-hospital deaths occurred in 7.9% (431/5,428), 5.4% (295/5,428), and 4.6% (251/5,428) of patients, respectively. Patients with high-risk conditions had a lower probability of survival than those without high-risk conditions (Fig. 1). Survival curves for selected subgroups of high-risk patients are shown in Supplemental Figs. 1–4.
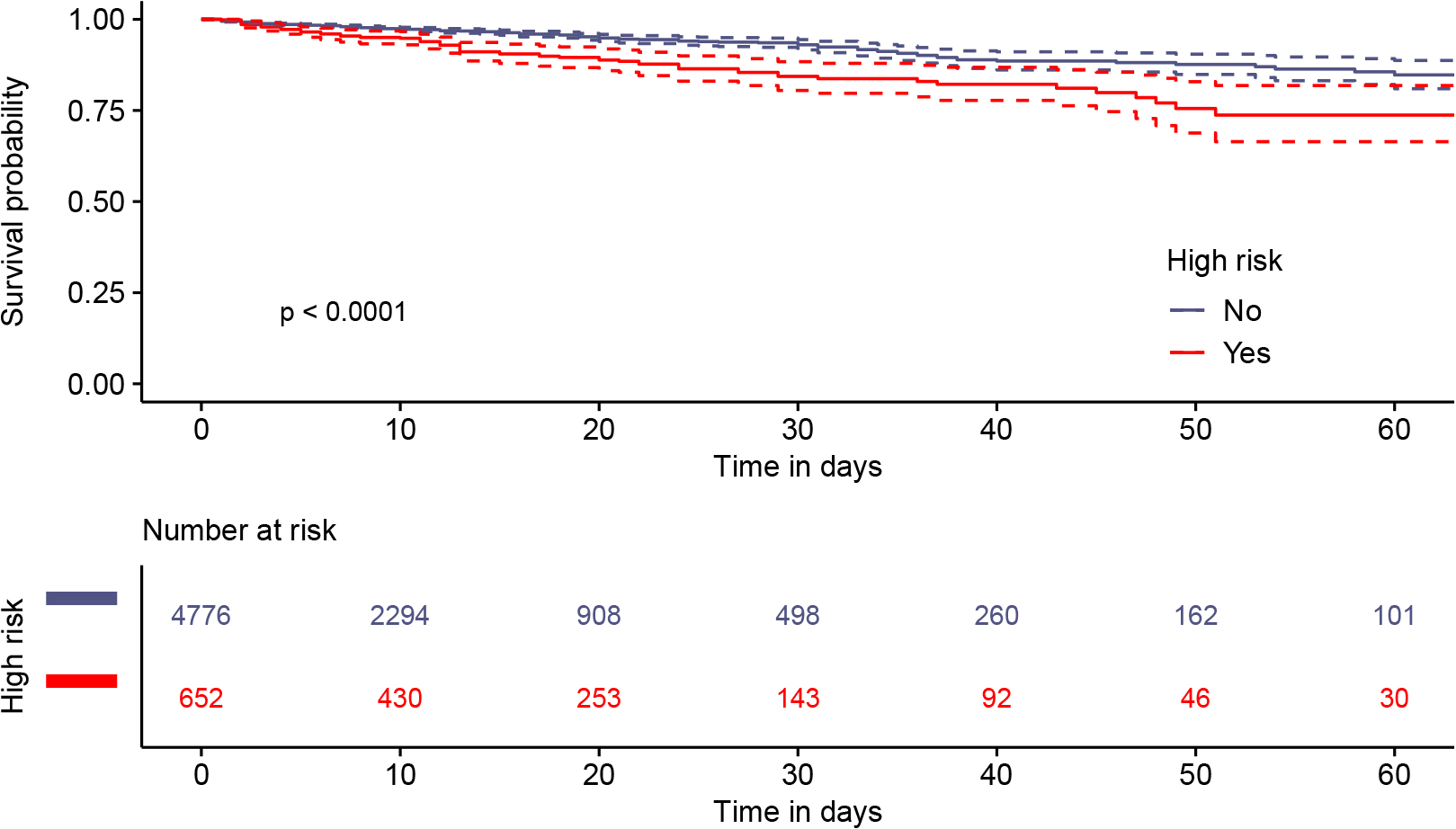
We report the descriptive epidemiology of COVID-19-related illness, including patient characteristics, care delivery, and outcomes in 2020. The data covered the first, second, and (part of the) third COVID-19 waves in Japan. Given that the epidemiology of COVID-19 has changed over time, our data should be viewed as a “snapshot” of the response to the COVID-19 pandemic in Japan during this period.
The threshold for hospitalization and discharge varied over the study period according to pandemic size, hospital capacity, and national/regional government policies. Therefore, the hospitalization data must be interpreted within this context. For example, in the early phase of the pandemic, all patients who tested positive for COVID-19 were hospitalized for isolation purposes [29], regardless of disease severity or patient risk factors. Although this policy was subsequently amended [30], it was strictly adhered to in some areas because of local government policies. Additionally, discharge of hospitalized patients was permitted 14 days or more after symptom onset. This period was shortened in June 2020 [31] to a minimum of 10 days from symptom onset for symptomatic inpatients and a minimum of 6 days from testing for asymptomatic patients. These regulations may explain why over 80% of inpatients were mild cases who did not require oxygen and why the median hospital stay was 10 days.
Repurposed drugs (also called repositioned or off-label use drugs) were the mainstay of COVID-19 pharmacological management in 2020, particularly for hospitalized patients with moderate to severe disease. Of these drugs, remdesivir was first authorized in May, followed by dexamethasone in July. Regarding other drugs, the “Guidance on the Management of Coronavirus Disease 2019 (COVID-19),” issued in September 2020 [32], listed tocilizumab, favipiravir, ciclesonide, nafamostat mesylate, and sarilumab as candidate drugs for COVID-19 under investigation; ivermectin and hydroxychloroquine were not mentioned. Although medication use may have been underreported in a minority of cases, the present study demonstrated that the use of repurposed drugs was largely evidence-based because unauthorized medication use was highly limited in 2020. This situation was in contrast to that in other countries, where repurposed drug use of ivermectin or hydroxychloroquine (inexpensive oral medications) was relatively common [33–35]. In some countries, these drugs were proposed as therapeutic options, such as the emergency use authorization of hydroxychloroquine in the United States (which was subsequently revoked) [36]. The infrequent use of these drugs in Japan may partly reflect the regulations of the public health insurance system, in which financial reimbursement for COVID-19 may not allow physicians to use unauthorized medications whose effectiveness is unproven or costs are high. Alternatively, because most patients in this study had mild disease, it is possible that they were judged as requiring only supportive care.
Compared with two previous registry-based observational studies in Japan, favipiravir use in our cohort was less frequent among hospitalized patients (4.7% vs 35.9%–71.9%) [11, 12]. This large discrepancy may be explained by differences in patient characteristics; one of these previous studies reported that favipiravir use was more common in severe cases requiring ventilator support [11]. It is also possible that healthcare providers in this previous study (which ended in July 2020) were encouraged to participate in clinical studies of favipiravir when they queried the Japanese government regarding the drug’s availability [11]. This may have prompted them to prescribe favipiravir to hospitalized patients, even though data on treatment efficacy were limited. As shown in this example, COVID-19 epidemiological data should be interpreted in the context of how and when data were collected during the ongoing pandemic.
Our findings could serve as a benchmark for future infectious disease pandemics, particularly during the early pandemic phase. First, this study provides data on how patient characteristics and care delivery changed over the course of the pandemic across different care settings. Our findings may help in the development of healthcare resource preparation and allocation plans. Second, epidemiological reports on the early phase of the COVID-19 pandemic often focused on the care of inpatients [37–41], particularly those with critical illnesses, whereas our study also included outpatients.
As the time evolved through each epidemic wave, the characteristics of inpatients appeared to shift toward more advanced age and more frequent comorbidities, which shift was not apparent in the outpatient setting (Supplemental Fig. 1). Although the reason for this was unclear, we speculate that the threshold for hospitalization has changed to more selective as the pandemic progressed, such reasons as the better understanding for high-risk condition of severe COVID-19 or hospital overload (though we did not have this metric in the database).
There are several study limitations. First, we lacked some clinical data, such as days of illness, signs and symptoms, vital signs, and body mass index data, of each patient. Therefore, it was not possible to classify disease severity; moreover, we were unable to determine whether adequate care was provided to each patient. During the COVID-19 pandemic, knowledge of patient care was updated rapidly. Despite this, the underuse of adequate therapy for severely ill patients has been reported in the United States, with remarkable variation among institutions [36]. It may be useful to explore whether such underuse of timely evidence-based treatments is also evident in other countries, including Japan. Second, we were unable to differentiate between regular and naïve users of prescribed medications. The study enrollment criteria did not define a data lookback period to ensure that all COVID-19 patients were included, and thus prescription history records were incomplete for a subset of patients. As a result, for example, statins may have been prescribed to regular statin users with hyperlipidemia or naïve users with COVID-19 to expect an anti-inflammatory effect [42]. However, we assume that the prescription of other drugs, such as remdesivir, favipiravir, and dexamethasone (particularly when used in the inpatient setting), was based on their likely indications for targeting COVID-19. Third, tracking patient outcomes was only possible within the same medical institutions. Therefore, the outcomes of outpatients who were subsequently hospitalized at other facilities or inpatients who were transferred to other hospitals may have been underestimated. Fourth, we could not access the results of the COVID-19 diagnostic tests (e.g., polymerase chain reaction); therefore, there is a possibility that COVID-19 was misclassified in some patients. However, public medical insurance payment for patients with COVID-19 is required by law. Thus, we believe it is unlikely that test-negative patients were classed as COVID-19 cases, even though we relied only on International Statistical Classification of Diseases and Related Health Problems, 10th revision codes to determine the diagnosis of COVID-19. Finally, medical institutions that submitted data to our database were not evenly distributed across Japan. Thus, our data may not be fully representative because of regional differences in practice patterns (e.g., prescription preferences), population composition, and hospital load.
In summary, we characterized both outpatients and inpatients with COVID-19 who were referred to multiple hospitals in 2020 in Japan in terms of background characteristics, treatments received, and resource utilization. We found that the use of unauthorized repurposed drugs was infrequent in our cohort. Although our findings present only a “snapshot” of the situation in Japan in 2020, they may be valuable for understanding the present pandemic and provide a benchmark for developing strategies for future infectious disease pandemics.
SI and TK are employees of Real World Data, Co., Ltd. KK received research funds from Eisai Co., Ltd., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Pfizer Inc., Stella Pharma Corporation, CMIC Co., Ltd., Suntory Beverage & Food Ltd., Mitsubishi Corporation, and Real World Data Co., Ltd.; consulting fees from LEBER Inc., JMDC Inc., Shin Nippon Biomedical Laboratories Ltd., Kaken Pharmaceutical Co., Ltd., and Advanced Medical Care Inc.; executive compensation from Cancer Intelligence Care Systems, Inc.; honorarium from Mitsubishi Chemical Holdings Corporation, Mitsubishi Corporation, Pharma Business Academy, and Toppan Inc.; and holds stock in Real World Data Co., Ltd. MT, NE, and TT have no competing interests relevant to this study.
This study was partly financially supported by Real World Data, Co., Ltd., including the publication fee. We thank Diane Williams, PhD, and Sarina Iwabuchi, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.