2025 Volume 81 Issue 3 Pages 152-163
2025 Volume 81 Issue 3 Pages 152-163
Overpopulated ungulates reduce the biomass of understory vegetation and promote the expansion of unpalatable plants in world forests. Such understory degradation may influence soil respiration (Rs) and heterotrophic respiration (Rh). Here, we examined this possibility in one cool-temperate forest in southern Kyushu, Japan. At the study site, the dominant understory vegetation, dwarf bamboo (Sasa; Sasamorpha borealis), has been patchy lost and replaced by an unpalatable shrub, Asebi (Pieris japonica), owing to sika deer feeding. We targeted three understory vegetation types: Sasa understory (SU), no understory (NU), and Asebi understory (AU). The Rs, Rh, soil temperature, and soil volumetric water content (SVWC) were measured at three points in each understory type using an automatic opening/closing chamber system from August 2022 to November 2023. We then evaluated biotic and abiotic factors, including surface litter amount, fine root biomass, and soil physiochemical properties, to examine their effects on the temperature sensitivity proxy (Q10) of Rs and Rh. Annual Rs and Rh were estimated using continuously measured soil temperature data and temperature-Rs and Rh relationships. The temporal variation of Rs and Rh was strongly affected by soil temperature and weakly by SVWC for all understory types. The spatial variation in Q10 of Rs and Rh was explained by fine root biomass and surface litter amount, respectively, regardless of understory type. Differences in annual Rs and Rh among understory types were comparable to those among measurement points in the same understory type. This was due to the similar soil temperature and Q10 of Rs and Rh among understory types. Thus, inner-site Rs and Rh variations could be generated by the spatial variations in soil biotic factors, regardless of understory vegetation type in our study site. This means our study cannot clearly verify whether overbrowsing increases Rs and Rh, highlighting the necessity for future research.
Sequestration of carbon dioxide (CO2), the main gas responsible for increasing air temperature (Friedlingstein et al., 2014), is a crucial function of forest ecosystems under global warming (Pan et al., 2024). The net carbon sink of global forests in the 2010s was estimated to be 3.5 Pg Cyr-1, offsetting approximately half of CO2 emissions from fossil fuels (Pan et al., 2024). The major pathway for CO2 efflux from forests is soil respiration (Rs), which consists of belowground autotrophic respiration (Ra) and heterotrophic respiration (Rh). The Rh represents respiration of microbes via the decomposition of surface litter and soil organic carbon (SOC), regulating the conversion of absorbed CO2 by vegetation into the soil reservoir. It is predicted that the ability of forests to absorb CO2 and store it as SOC is weakened through increased intensity of disturbances (Pan et al., 2024). One concern about the intensification of disturbances is the recent increase in ungulate populations in forests worldwide (Wilson and MacLeod, 1991; Coomes et al., 2003; Takatsuki, 2009; Tape et al., 2016; Guerisoli and Pereira, 2020).
Overpopulation of ungulates leads to excessive and prolonged understory vegetation feeding (hereafter referred to as overbrowsing) (Guerisoli and Pereira, 2020). Overbrowsing reduces the biomass and species diversity of understory vegetation (Hernández and Silva-Pando, 1996; Horsley et al., 2003; Kato and Okuyama, 2004; Tremblay et al., 2006; Suzuki et al., 2008; Harada et al., 2020). In addition, overbrowsing increases the abundance of plant species that are unpalatable for an ungulate diet (Enoki et al., 2017; Abe et al., 2024b; Tokumoto and Katayama, 2024). Such understory degradation potentially degrades forest carbon sequestration, for example, through an increase in carbon emissions from the forest (Ramirez et al., 2018; Schmitz et al., 2018; Forbes et al., 2019; Leroux et al., 2020). Further research examining this possibility is necessary to implement forest management practices that reduce carbon emissions under global warming and overbrowsing.
Overbrowsing-induced understory alteration occurs heterogeneously within a landscape and a stand (Lilleeng et al., 2016; Enoki et al., 2017; Abe et al., 2024b; Tokumoto and Katayama, 2024). Comparison of different understory types within a stand is the first step in evaluating whether overbrowsing-induced understory loss and replacement affect Rs and Rh under fixed climatic and topographic conditions. In addition, examination of biotic and abiotic factors that drive Rs and Rh is useful for such comparison. This is because soil temperature and moisture are primary drivers that determine temporal variation of Rs and Rh (e.g., Lloyd and Taylor, 1994; Saiz et al., 2006; Webster et al., 2009). Temperature and moisture sensitivities of Rs and Rh vary with biotic and abiotic factors in soils, such as root biomass and production, soil bulk density (BD), and soil carbon concentration (SC) (Bond-Lamberty and Thomson, 2010; Hursh et al., 2017; Tang et al., 2020; Jian et al., 2022). These factors are modified by overbrowsing-induced understory loss and replacement (Niwa et al., 2011; Tokumoto and Katayama, 2024; Tokumoto et al., 2024; Kooijman and Smit, 2001; Kawakami et al., 2020a, 2020b; Katayama et al., 2023). Thus, exploration of the biotic and abiotic factors that determine the sensitivities of Rs and Rh can help to infer the effect of understory alteration on Rs and Rh.
Currently, the population size of sika deer (Cervus nippon) has reached its historically highest level in the Japanese archipelago (Iijima et al., 2023). In the southern part of Kyushu Island, the dominant understory vegetation, dwarf bamboo (Sasa; Sasamorpha borealis) (Fig. 1a), has been decreasing and disappearing as a result of overbrowsing since the 1980s (Fig. 1b) (Saruki et al., 2004). In addition, overbrowsing has led to the replacement of Sasa by the unpalatable shrub, Asebi (Pieris japonica) (Fig. 1c) (Enoki et al., 2017; Tokumoto and Katayama, 2024). In this study, we examined whether the understory loss and species replacement caused by sika deer affect the Rs or Rh and their sensitivity to soil temperature or moisture. To achieve this aim, we conducted field measurements of Rs and Rh in one cool-temperate forest with three types of understory vegetation (i.e., Sasa understory, no understory, and Asebi understory). Using measured data, we modeled the sensitivity of Rs and Rh to soil temperature and moisture. We then estimated the annual amount of Rs and Rh. In addition, we compared the obtained temperature sensitivity proxy (Q10) of Rs and Rh with potential biotic and abiotic factors.

Fig. 1. Location of the study site (a), map of the study site (b), and understory vegetation types at the study site (c-e). In panel b, dots represent the CO2 efflux measurement points. These points comprise pairs of measurement locations for total soil respiration (Rs) and heterotrophic respiration (Rh). Circles represent the points for dwarf bamboo (Sasa; Sasamorpha borealis) understory (SU, panel c). Triangles represent the points for no understory (NU, panel d). Squares represent the points for an unpalatable shrub, Asebi (Pieris japonica) understory (AU, panel e). Red symbols indicate the points for continuous soil temperature recording. In panel a, symbols other than the present study site (cross) indicate the stands where continuous soil temperature data for SU (circle), NU (triangle), and AU (square) were used to complement the missing soil temperature data for the present study site (see section 2.5).
This study was conducted in the Kyushu University Shiiba Research Forest (SRF), located in the southern part of Kyushu Island (32°20′53″N, 131°5′32″E, 880 m above sea level) (Fig. 1a). The mean annual temperate (MAT) and precipitation (MAP) in the study area were 10.8°C and 3207.9 mm, respectively (DEIMS-SDR, 2021). The study site was on flat terrain with a slope of <5 degrees. We established a study plot with an area of 600 m2 on March 2, 2024 (Fig. 1b). In the study plot, species name and diameter at breast height (DBH) were recorded for overstory trees with DBH >3 cm. The mean and standard deviation (SD) of DBH were 18.4 ± 9.7 cm. The stem density was 683.6 stems ha-1, and the basal area was 23.0 m2 ha-1. The dominant species were Quercus variabilis, Q. crispula, and Q. serrata. These three species comprised 85.4% of the stem density and 83.6% of the basal area.
Before overbrowsing, the understory vegetation in SRF was entirely covered by Sasa (i.e., S. borealis). Because of overbrowsing since the 1980s, Sasa has decreased (Saruki et al., 2004), and the population of Asebi has increased (Enoki et al., 2017; Ichihashi and Katayama, 2024; Tokumoto and Katayama, 2024). We targeted three understory types within the study plot: Sasa understory (SU, Fig. 1c), no understory (NU, Fig. 1d), and Asebi understory (AU, Fig. 1e). SU was located within areas enclosed by a deer exclusion fence and was considered to be the baseline understory type (Abe et al., 2024b; Tokumoto et al., 2024). NU and AU were located outside the fenced areas and were considered to be overbrowsing-induced understory types (Abe et al., 2024b). AU developed from NU with high light availability due to the expansion of Asebi (Tokumoto and Katayama, 2024).
In the study plot, we established three CO2 efflux measurement points for Rs and Rh in each understory type (i.e., three understory types × three-point replications × set of Rs and Rh = 18 locations) on March 16, 2022 (Fig. 1b). We installed short polyvinyl chloride (PVC) collars at each point to measure Rs. We employed a micro-trenching method to measure Rh (Riutta et al., 2021); for this method, tall PVC collars were installed at each measurement point. The diameter of the PVC collars was 14.5 cm (i.e., 165 cm2 in area), with a height of 10 cm for the short collars and 45 cm for the tall collars. Installed PVC collars were exposed 5 cm above the ground surface. The roots inside the tall PVC collars were cut to install the tall PVC collars. Hence, Rh in this study excludes CO2 efflux from living roots and the associated rhizosphere at 0-40 cm depth (Riutta et al., 2021). The collars were allowed to stabilize for 18 weeks before the data collection started. Previous studies that used the micro-trenching method have shown that CO2 efflux in tall PVC collars stabilized within 1 week (Sapronov and Kuzyakov, 2007; Thurgood et al., 2014; Riutta et al., 2021).
2.2. Vegetation and soil censusTo estimate understory vegetation biomass, we installed a 0.5 m × 0.5 m frame surrounding each CO2 efflux measurement point. On March 2, 2024, we measured the number of culms of Sasa, the culm height of Sasa, and the diameter at 5 cm height (D5, cm) of Asebi inside the frame. The biomass of Sasa was estimated using the following equation:

where B is the biomass of Sasa (g m-2), N is the number of culms of Sasa (m-2), and H is the culm height of Sasa (m). This equation was created from the results of understory harvesting survey in SRF (Abe et al., 2024b) (Fig. S1). The biomass of Asebi was estimated using an allometric equation established in SRF (Ichihashi and Katayama, 2024):

where W is the individual biomass of Asebi (g). The total value of W in each frame was divided by the frame size (0.025 m2) to obtain area-based biomass (g m-2).
We collected soil samples on March 2, 2024, to evaluate biotic and abiotic factors that influence Q10 of Rs and Rh. The target biotic and abiotic factors were surface litter amount (g m-2), fine root biomass (g m-2), SOC amount (g C m-2), BD (g m-3), and SC (g C g-1). The surface litter included leaf litter and fine woody debris with a diameter of approximately <3 cm (Abe et al, 2022b). Fine roots included all living plant roots with a diameter of <2 mm. We sampled surface litter inside 0.3 m × 0.3 m frames placed near the measurement points. Surface litter was separated into Sasa, Asebi, and other tree species. Surface litter that had decomposed to inseparability was classified as inseparable. Surface litter was oven-dried at 70°C for 48 h and weighed. We sampled soil cores to determine fine root biomass, BD, and SC. This was performed around all measurement points at 0-5 cm and 5-10 cm depths. After removal of the surface litter, we used a 100 cm3 syringe to collect soil cores in three positions at each measurement point. The soil core samples were pooled for each measurement point and depth (i.e., 300 cm3 soils for each measurement point at both 0-5 cm and 5-10 cm depths). The pooled soil was sieved through a 2 mm mesh, and then mineral soil and fine roots were separated. The fine roots were further separated into Sasa, Asebi, and other tree species. No inseparable roots were present. Mineral soil and fine roots were oven-dried at 70°C for 48 h and weighed. BD was determined by dividing the mass of mineral soil (g) by the sample volume (0.0003 m3). SC was measured using a C/N corder (Macro Corder JM1000CN, J-Science Lab Co., Kyoto, Japan). The SOC amount was calculated by multiplying BD, SC, and the sampling depth of each soil layer (0.05 m).
2.3. Soil respiration measurementWe measured Rs and Rh at each measurement point using a closed static chamber (HL-1019A, Fuxin Gongda Hualian Technology Co., Ltd., Liaoning, China) (Kuriyama et al., 2021; Wang et al., 2022). The chamber system consisted of a cylindrical chamber (soil exposure area: 165 cm2, chamber volume: 3300 cm3), an infrared gas analyzer, and a fan that circulates the air in the chamber in one unit. A thermometer measured the gas temperature in the chamber. Measurements of CO2 concentration and gas temperature were recorded at 3-second intervals over 7.5 min. The CO2 efflux (F, µmol m-2 s-1) was calculated as follows:

where dc/dt is the CO2 concentration increment per second (μmol mol-1 s-1), Vs is the volume of the chamber (3.3 L), Va is the standard molar volume of the atmosphere (22.4 L mol-1), Ta is the gas temperature (°C), and A is the soil exposure area (0.0165 m2). After the measurement period, the movable partition opened and automatically exhausted the air from the chamber. The system automatically recorded CO2 efflux at 20-minute intervals, including the exhaust period. In each recording period, soil temperature (°C) and soil volumetric water content (SVWC, %) at 5 cm depth near the chamber were automatically measured from a thermometer and a time domain reflectometer sensor. Measurements were conducted during six campaigns from 2022 to 2023 (August 5 to October 6, 2022; November 14 to December 4, 2022; February 14 to February 28, 2023; March 14 to April 10, 2023; July 10 to August 31, 2023; and October 30 to November 8, 2023). In each campaign, two chambers were used to measure CO2 efflux at one short PVC collar and one tall PVC collar at each measurement point. The two chambers were relocated to different points every 1-2 days until measurements were completed at all points. Thus, CO2 efflux at each point was recorded for at least 24 h in each measurement campaign. During the campaign, CO2 efflux measurement was suspended when it became difficult to conduct, such as under heavy rainfall and when road access was blocked.
2.4. Soil respiration modelingWe examined the relationships of Rs and Rh with soil temperature and SVWC for each understory type. This was done using three empirical equations. The first equation expresses the exponential relationship using soil temperature (Lloyd and Taylor, 1994):

where T is soil temperature (°C) at 5 cm depth, and a and b are constants. The second equation expresses quadric relationships using SVWC (Saiz et al., 2006):

where θ is SVWC (%) at 5 cm depth, and c, d, and f are constants. The third equation expresses the exponential power relationships using T and θ (Saiz et al., 2006):

where g, h, and i are constants. To estimate the constants in Eqs. 4-6, non-linear mixed-effect models (Lindstrom and Bates, 1990) were applied to pooled data from the three measurement points for each understory type. Thus, all constants in Eqs. 4-6 consisted of fixed and random effects. Fixed effects were expressed as representative constants in each understory type. Random effects were assigned to differences in measurement points in each understory type. Constants were fitted using a maximum likelihood procedure. The goodness of fit was evaluated with the Akaike information criterion (AIC), and the coefficient of determination was explained by fixed effects (Marginal R2) (Spiess and Neumeyer, 2010). The analyses were conducted using R software version 4.2.2 for Windows 10 x64 (R Core Team, 2024) with the packages “nlme” (Pinheiro et al., 2017) and “nlraa” (Miguez, 2021).
We calculated Q10 to determine the temperature sensitivity of Rs and Rh as follows (Lloyd and Taylor, 1994):

where b is the constant in Eq. 4. Representative Q10 in each understory type was calculated based solely from the fixed effects of constant b. In addition, the spatial variation of Q10 was evaluated as the SD of Q10 between measurement points in each understory type. Q10 in each measurement point was calculated from constant b at each measurement point, which is the sum of fixed effects and point-specific random effects.
2.5. Annual soil respiration estimationTo estimate annual Rs and Rh, continuous records of soil temperature were substituted into the fitted temperature-driven soil respiration model (i.e., Eq. 4). The models including SVWC (i.e., Eqs. 5 and 6) were not used for this estimation because of the lack of continuous SVWC data and the lower contribution of SVWC to the temporal dynamics of Rs and Rh in this study (see 3.2). Using the same procedure for calculating Q10 (see 2.4), we calculated the representative annual Rs and Rh based solely on the fixed effect of the model. The extent of variation among measurement points (i.e., SD of annual Ra and Rh) were calculated from the fixed effect and point-specific random effects of the model. The soil temperature at 5 cm depth was recorded at one measurement point for each understory type (Fig. 1b). The soil temperature was recorded using a thermometer (TMS4, TOMST, Praha, Czech Republic) at 30-minute intervals from March 14 to July 14, 2024. Because the recording duration of these data was less than one year, the data for the missing period (July 15, 2023 to March 13, 2024) were complemented as follows. We utilized soil temperature data for SU, NU, and AU in other stands recorded by Abe et al. (2024c) (Fig. 1a). The utilized data were converted to adjust the soil temperature at the present study site. The conversion formula was created based on a linear regression of the data for the period from March 15 to May 12, 2024, when the recording periods overlapped (Fig. S2). Since the unit of the predicted CO2 efflux is µmol CO2 m-2 s-1, it was multiplied by 1800 seconds to convert it to a 30-minute cumulative value (µmol CO2 m-2 30 min-1) and then summed to obtain the annual cumulative value (µmol CO2 m-2 yr-1). Consequently, the estimated annual cumulative value was converted to a weight-based value (g C m-2 yr-1) using the molecular weight of C (12.01 g mol-1).
2.6. Statistical tests and comparison to global soil respiration datasetsWe used the Tukey honest significant differences test to assess whether understory vegetation biomass, surface litter amount, fine root biomass, SOM amount, BD, and SC varied among different understory types. In addition, the effects of these variables on the Q10 of Rs and Rh were evaluated by linear regression analysis. This regression analysis targeted Q10 values for each measurement point. The significance threshold for statistical tests was set at p < 0.05. These analyses were conducted in R software with packages “stats” (R Core Team, 2024) and “multicomp” (Bretz et al., 2010).
We compared our annual Rs and Rh with those reported for other forest sites recorded in the soil respiration data base (SRDB) version 5 (Jian et al., 2021). For this comparison, we extracted records from the SRDB where the Ecosystem type was “Forest” and the Ecosystem state was “Natural”. The relationship between Rs and Rh for MAT and MAP was plotted based on the obtained data, in addition to our average value of annual Rs and Rh in each understory type (see Fig. S6). Further, because Rs and Rh are primary influenced by temperature (e.g., Lloyd and Taylor, 1994), we limited the comparison to sites with a similar range of MAT. Specifically, we used data within a ±25% range of our study site’s MAT (10.8°C × 0.25, resulting in a range of 8.1-13.5°C).
The understory vegetation biomass in SU (3382.1 ± 728.5 g m-2) was 26-fold higher than that in NU (128.7 ± 222.8) and 5-fold higher than that in AU (653.7 ± 306.4) (Table 1, Table S1). No differences in surface litter amount were detected among SU (1197.3 ± 56.9 g m-2), NU (984.1 ± 265.5), and AU (1011.2 ± 146.1). At each measurement point, overstory trees contributed 92%-98%, 100%, and 51%-84% of the total surface litter in SU, NU, and AU, respectively (Fig. S3). No differences in fine root biomass were observed at any depth among understory types (e.g., biomass at 0-10 cm depth was 215.6 ± 51.7 g m-2 in SU, 273.9 ± 99.2 in NU, and 291.1 ± 155.9 in AU). Nevertheless, Sasa and Asebi contributed 23%-35% and 42%-63% of the fine root biomass at 0-10 cm depth in SU and AU, respectively (Fig. S4). The SOC at 0-5 cm and 0-10 cm depths in NU was 1.51- and 1.15-fold higher than in SU and AU, respectively (e.g., SOC at 0-10 cm depth was 1843.4 ± 253.9 g m-2 in SU, 2781.0 ± 384.2 in NU, and 2412.6 ± 199.6 in AU). This was due to the relatively higher BD in NU than in SU and AU, e.g., BD at 0-5 cm depth was highest in NU (0.16 ± 0.0088 g cm-3), followed by AU (0.13 ± 0.017) and SU (0.11 ± 0.026). However, differences in BD were not significant at any depth. In addition, no differences in SC were detected at any depth among SU, NU, and AU.
Table 1. Mean and standard deviation (SD) of understory vegetation biomass, fine root biomass, and soil properties in Sasa understory (SU), no understory (NU), and Asebi understory (AU). Different lowercase letters a and b indicate a significant difference (p < 0.05, two-sided Tukey honest significant differences test).
 3.2. Estimated soil temperature and moisture models for soil respiration
3.2. Estimated soil temperature and moisture models for soil respiration
A consistent positive exponential response of Rs and Rh to soil temperature was observed for all understory types (Fig. 2). The R2 values for the temperature model (i.e., Eq. 4) ranged from 0.84 to 0.92, suggesting that most of the variation in Rs and Rh was explained by soil temperature (Table 2). Unimodal responses of Rs and Rh to SVWC were detected, except for Rh in SU and Rs in AU (Fig. S5). However, the R2 values for the SVWC model (i.e., Eq. 5) were less than 0.5 (0.015 to 0.48; Table 2). Furthermore, the AIC and R2 values for the hybrid model of temperature and SVWC (i.e., Eq. 6) did not differ from those of the temperature model (e.g., the results in SU had AIC = -5439.92 and R2 = 0.84 for the temperature model, and AIC = -5611.54 and R2 = 0.88 for the hybrid model; Table 2).

Fig. 2. Response of soil respiration efflux (μmol m-2 s-1) to soil temperature at 0-5 cm depth. Data points represent Rs and Rh. Symbols (circles, triangles, and squares) indicate the different measurement points. The solid line indicates the regression line obtained from the fixed effect of the non-linear mixed-effect model with random effects as replicates of the measurement points. The dotted line indicates the regression line at each measurement point (i.e., fixed effect + point-specific random effect).
Table 2. Estimated constants and their SE as well as SD of random effects (RE). The value of n indicates the number of data points. The model terms T, θ, and T & θ indicate soil temperature, soil volumetric water content, and hybrid temperature and water models, respectively (see Eqs. 4-6). Values of R2 are the marginal R2 of the model.

The Q10 calculated from the fixed effect ± SD of the random effects (Table 2) were as follows. The Q10 of Rs was 2.42 ± 0.31 for SU, 2.50 ± 0.19 for NU, and 2.60 ± 0.34 for AU. The Q10 of Rh was 2.73 ± 0.07 for SU, 3.00 ± 0.52 for NU, and 3.17 ± 0.33 for AU. For Rs and Rh, differences in Q10 among understory types were only as large as the SD of the random effect (i.e., the variation among each measurement point).
Based on the temperature model (i.e., Eq. 4, Table 2), annual Rs and Rh and their seasonable trends were predicted (Fig. 3). The soil temperature in SU, NU, and AU showed consistent seasonal variation: it increased from March, peaked in July, and then decreased to the minimum value from January to March. No clear differences in soil temperature were observed among understory types in any season. The seasonal variation of Rs and Rh was similar to that of soil temperature. Annual Rs calculated from the fixed effect ± SD of the random effects was 210.8 ± 77.4 g C m-2 yr-1 for SU, 206.9 ± 66.9 g C m-2 yr-1 for NU, and 183.9 ± 69.4 g C m-2 yr-1 for AU. The differences in annual Rs among understory types were as much as 26.9 g C m-2 yr-1, lower than the SD of annual Rs in the same understory type (i.e., the variation among each measurement point). Similarly, the differences in annual Rh among understory types were lower than the SD of annual Rh in the same vegetation type (SU = 149.1 ± 11.9, NU = 120.5 ± 78.4, and AU = 147.2 ± 37.1 g C m-2 yr-1).

Fig. 3. Seasonal trends for soil temperature (Ts, °C), Rs (μmol m-2 s-1), and Rh (μmol m-2 s-1). Lines within shaded areas of Rs and Rh indicate predicted Rs and Rh from the fixed effect with SD of the random effect, respectively. The values inside the panel show the annual Rs and Rh (g C m-2 yr-1) calculated from the fixed effect ± SD of the random effect. The vertical line indicates the date to be March 14, 2024. The soil temperature after March 14, 2024 was recorded at the present study site. The soil temperature before March 14, 2024 was derived from interpolated data (see section 2.5).
Table 3 shows the results of regression analysis for the Q10 of Rs and Rh. Relationships with p < 0.05 were detected for surface litter amount and fine root biomass. Surface litter amount was positively related to the Q10 of Rh (R2 = 0.59, p = 0.015) (Fig. 4). Fine root biomass at 0-10 cm and 0-5 cm depths was positively related to the Q10 of Rs (0-10 cm; R2 = 0.69, p = 0.0060, 0-5 cm; R2 = 0.47, p = 0.041) (Fig. 5).
Table 3. Results of regression analysis for Q10 of Rs and Rh. The p values show the significance of the slope value. Results with p < 0.05 are shown in boldface. The ‘+’ symbol in the R2 column indicates a significant positive effect.
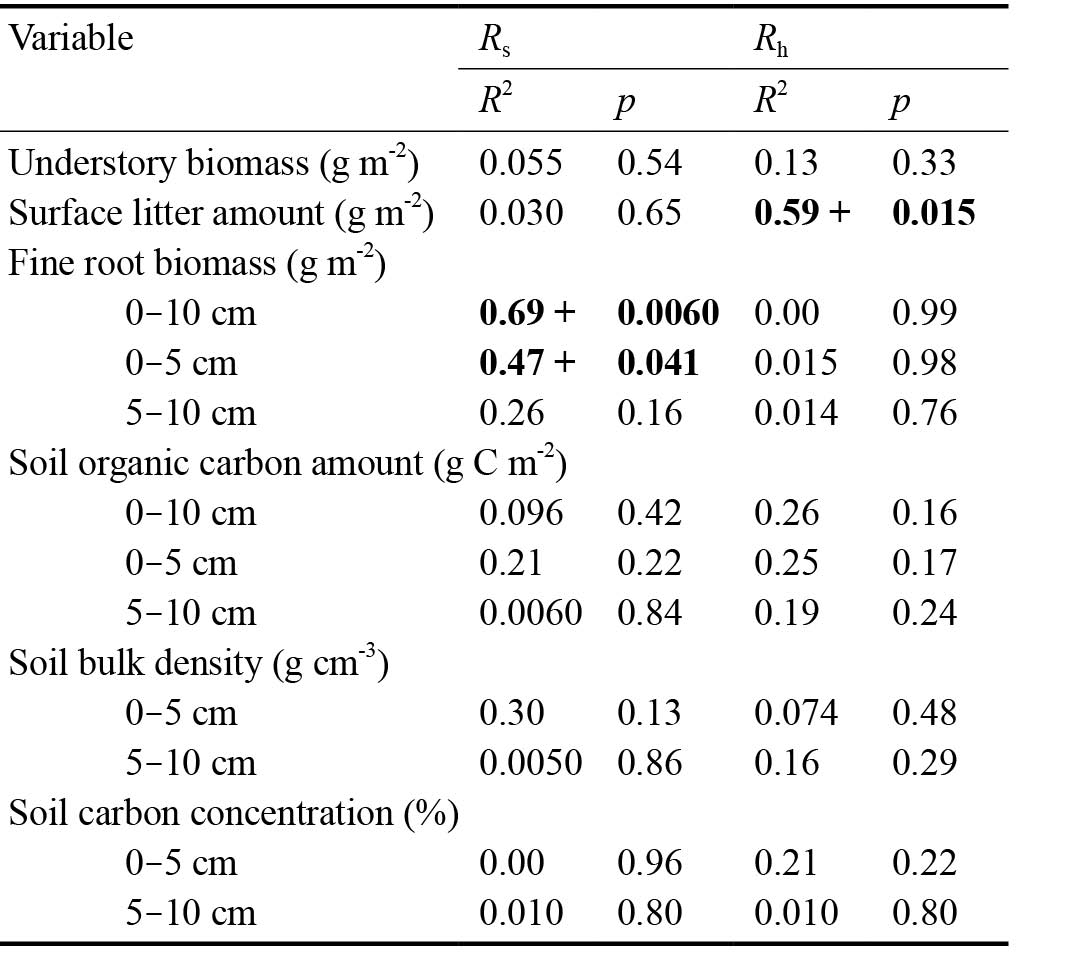

Fig. 4. Relationships between surface litter amount and Q10 of Rh. Data points represent the results for each measurement point. Symbols represent understory types. The solid line indicates the significant regression line, and the gray area indicates the 95% confidence interval.

Fig. 5. Relationships between fine root biomass at 0-10 cm depth and Q10 of Rs. Data points represent the results for each measurement point. Symbols represent understory types. The solid line indicates the significant regression line, and the gray area indicates the 95% confidence interval.
The temporal variation of Rs and Rh was affected strongly by soil temperature and weakly by SVWC (Table 2). This result was consistent with most previous studies that indicated the temporal variation of Rs and Rh is controlled primarily by soil temperature (e.g., Lloyd and Taylor, 1994; Webster et al., 2009; Chen et al., 2020). The possible reason for the lower sensitivity of SVWC is the climatic conditions at the present study site. The sensitivities of Rs and Rh to SVWC are higher in dry areas than in wet areas (Manzoni et al., 2012; Liu et al., 2016; Morris et al., 2022). For instance, in arid grasslands, Rs is more sensitive to SVWC than to soil temperature; therefore, ungulate browsing significantly alters Rs sensitivity to SVWC (Jia et al., 2006). The MAP in the present study area was 3207.9 mm, indicating that the soil is always well-moistened. Therefore, the present study area showed lower sensitivities of Rs and Rh to SVWC despite the different understory types.No clear differences in annual Rs and Rh were detected among SU, NU, and AU (Fig. 3). This was because of the similarity of both annual soil temperature trends (Fig. 3) and sensitivities of Rs and Rh to temperature (Table 2, Fig. 2). Regarding the annual soil temperature trend, the data were obtained from one location for each understory type and were complemented from autumn to winter. For this reason, it is difficult to speculate on the cause of the similarity of soil temperature in the present study, and further measurements are required. The similarity in Q10 of Rs and Rh between SU and NU accords with previous findings that understory vegetation removal does not change the Q10 in other temperate forests (Yashiro et al., 2012; Li et al., 2019). The similarity in Q10 of Rs and Rh in the present study site may be explained by the lack of differences in biotic and abiotic factors driving Q10, i.e., surface litter amount and fine root biomass (Table 1; Table 3).
4.2. Underlying factors influencing Q10 in different understory typesSurface litter amount did not differ among understory types (Table 1) and was mainly composed of tree litter (Fig. S3). Surface litter amount positively affected the spatial variation in Q10 of Rh (Fig. 4). This positive effect is consistent with previous reports that litter addition increases, and litter removal decreases, the Q10 of Rh (Liu et al., 2022; Zhuang et al., 2023). Surface litter provides additional substrate and increases microbial biomass, which in turn enhances the Q10 of Rh (Zhuang et al., 2023). The priming effect, whereby the addition of fresh organic matter stimulates the decomposition of older organic matter, can also increase the Q10 of Rh (Liu et al., 2020). Although the surface litter of Sasa and Asebi were expected to differ in degradability from the litter of tree species (Watanabe et al., 2013; Tokumoto and Katayama, 2024), the effect might be minimal because Sasa and Asebi accounted for a small percentage of the surface litter in SU and AU (Fig. S3).
Although root species composition differed with understory type (Fig. S4), total fine root biomass did not change (Table 1). Fine root biomass positively affected the spatial variation in Q10 of Rs (Fig. 5). This positive effect is consistent with previous findings that fine root biomass explains the spatial variability of Q10 in Rs (Luan et al., 2013; Han and Jin, 2018). Root biomass explains the spatial variation in Rs (Behera et al., 1990; Rodeghiero and Cescatti, 2006; Ceccon et al., 2011; Comeau et al., 2018; Abe Y et al., 2022). Furthermore, higher root biomass can indirectly contribute to Rs through the decomposition of dead roots (Saha et al., 2023) and stimulation of SOM decomposition (Adamczyk et al., 2019). Thus, the present results suggested that the variability of the Q10 of Rs at the study site was mainly regulated by fine root biomass and was not affected by root species composition.
The topography may affect the differences in surface litter amount among understory types but not in fine root biomass. Abe et al. (2024b) conducted a vegetation and soil properties census for SU, NU, and AU in forests 3-10 km distant from the present study site. This previous study reported no change in understory vegetation biomass and fine root biomass, supporting the present results and inferences. However, the surface litter amount was reduced because of overbrowsing in the previous study site. This finding was inconsistent with the present results. The previous study site was located on a steep slope of 7.9-37.3°, on which severe soil erosion had occurred (Abe et al., 2022a, 2024a). In contrast, the present study site was located on flat terrain with a slope of less than 5°, where reduction in surface litter associated with soil erosion was unlikely. This suggests that the impact of alteration in understory vegetation by overbrowsing on the Q10 of Rh may be mediated by site erodibility. In particular, the Q10 of Rh on steep slope terrain may decrease because of the loss of surface litter by soil erosion.
4.3. Limitation and future perspectiveIn this study, we could not find clear differences in the annual Rs and Rh among three understory vegetation types. Besides the variability in Q10, two reasons may explain this: the low annual Rs and Rh values at our study site and the limited statistical power.
The average value of annual Rs obtained in the present study ranged from 183.9 to 210.8 g C m-2 yr-1 (Fig. 3). The annual Rh ranged from 120.5 to 149.1 g C m-2 yr-1 (Fig. 3). When compared with Rs and Rh recorded from SRDB, our results (MAT = 10.8°C, MAP = 3207.9 mm) fell towards the lower end of the range for sites with similar MAT or MAP (Fig. S6). For example, the mean ± SD of annual Rs in sites with a MAT of 8.1-13.5°C was 819.8 ± 407.8 g C m-2 yr-1, with a minimum and maximum range of 32.0-1907.0 g C m-2 yr-1 (Fig. S6a). Similarly, the mean ± SD of annual Rh was 462.65 ± 375.7 g C m-2 yr-1, with a range of 32.0-1907.01 g C m-2 yr-1 (Fig. S6c). The relatively low Rs and Rh values at our study site suggest that it might be difficult to clearly detect differences in the absolute values of annual Rs and Rh among the understory vegetation types. Therefore, conducting similar experiments at sites with potentially higher Rs and Rh might yield different results than those observed in the present study.
Our results are based on findings in a single stand on flat terrain and moist soils. In addition, this study was only conducted measurements with three-point replicates per understory type, lacking higher statical power. Therefore, it remains uncertain whether climatic, topographic, and soil biotic/abiotic factors mediate the causal inference of understory types on Rs and Rh. It is essential to generalize the present results by accumulating site-based knowledge, such as conducting similar comparisons of the present study in multiple stands with different site characteristics.
We found the inner-site variations in Rs and Rh could be generated by the spatial variations of soil biotic factors, regardless of understory vegetation types in our study site. There were no obvious differences in Q10 of Rs and Rh as well as soil temperature among understory types. The Q10 of Rs and Rh were related to surface litter amount and fine root biomass; these biotic factors had no clear differences among understory types. However, it is important to acknowledge that the findings of the present study are based on measurements from a single, relatively small forest stand with a limited number of sampling points. Consequently, the present study cannot definitively rule out the potential for overbrowsing-induced understory degradation to lead to increased Rs and Rh. Future research is necessary to verify whether understory degradation subsequently impair forest carbon sequestration through increased Rs and Rh. This future research will contribute to identifying the site characteristics of stands that emit large amounts of carbon in the context of global warming and overbrowsing.
This work was supported by the JSPS KAKENHI (grant numbers JP22KJ2456, JP21H05316, JP20H02684). Inaddition, this publication fee was supported by the Japan Society for the Promotion of Science (grant number 23HP2004). We acknowledge the staff of the SRF for permitting us to conduct the research, and managing the study site. We also thank Mr. Zhouqiang Li and Mr. Dongchuan Fu for their kind assistance of soil sampling and vegetation measurement. We thank Robert McKenzie, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AH contributed to conceptualization, funding acquisition, investigation, data analysis, and drafted the original manuscript. KT contributed to funding acquisition, investigation, and revision/editing of the manuscript. KA contributed to the supervision, conceptualization, funding acquisition, investigation, and revision/editing of the manuscript.
The point-level data, including Q10 of Ra and Rh, as well as understory conditions, are available in Table S1 in supplementary materials. More detailed data will be made available upon request.