2019 Volume 19 Issue 1 Pages 1-8
2019 Volume 19 Issue 1 Pages 1-8
Purpose: Several hydrophilic and hydrophobic approaches have been developed to prevent food debris and plaque accumulation on the denture surface. In this study, we evaluated the possibility of developing a high-functioning self-cleaning denture. Materials and Methods: Denture base resin specimens were divided into the following groups based on the type of surface treatment: control (no treatment); hydrophilic (UV irradiation or tribochemical coating); and hydrophobic (plasma chemical vapor deposition, CVD). Surface analyses were conducted using X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy, and atomic force microscopy. Physical strength was evaluated using the three point bending test. Data were analyzed using one-way analysis of variance followed by multiple comparisons. Results: The self-cleaning effects of the hydrophilic treatments were unsatisfactory; alternatively, the hydrophobicity, mechanical properties, and self-cleansing ability of the CVD specimens were significantly high (p < 0.05). Conclusion: Hydrophobic surface modifications effectively improved the water repelling properties of the resin without compromising its mechanical properties.
Aspiration pneumonia is the leading cause of death in the elderly. It is mainly caused by infectious diseases via oral indigenous bacteria as a result of poor oral hygine. It has been reported that approximately 16% of denture wearers use appropriate methods to clean their dentures [1,2]. Poly(methyl methacrylate) (PMMA) resin is commonly used as a denture base material; however, the water absorbing property of this resin encourages the adhesion of plaque along with food debris and salivary proteins on the surface of the denture. Denture cleaning is rarely performed correctly even after providing detailed instructions to the denture wearers. This may be due to the fact that older people with decreased vision and/or motor skills may find it difficult to clean small and complex shaped dentures using special cleaners and brushes after every meal. Thus, insufficient cleaning results in denture plaque accumulation on the surface of the dentures leading to the occurrence of denture stomatitis and aspiration pneumonia [3].
Sawada et al. [4] developed a self-cleaning denture by adding a photo catalyst material, fluorapatite-titania (Fap-TiO2) to the denture base acrylic resin and applying UV irradiation for 6 h per day. However, it is important to note that the mechanical strength of acrylic resin could be compromised due to the breakage of bonds by radicals produced after UV irradiation or addition of TiO2, in addition to the promotion of cross-linking reactions due to the formation of intermolecular bonds at the cutting site [5,6].
Several hydrophilic and hydrophobic approaches have been developed to prevent the adhesion of stains on the surface of the denture. Chemical surface alteration by UV irradiation [7] and mechanical and structural changes using tribochemical methods have been used to improve the hydrophilic characteristics of the denture surface. Tribochemistry is a biomimetic technology used for the prevention of staining in fractal structures with numerous regular micro-asperities [8]. Because of the hydrophilicity of these structures, residual food debris, plaque, and stains can be easily removed even with gentle water pressure. The plasma chemical vapor deposition (CVD) method was propsed to obtain anti-staining effects based on its hydrophobic characteristics [9,10]. This method is thought to affect cell adhesion on the surface via prohibition of cell division and growth thereby exhibiting superior biocompatibility [11].
In the present study, the possibility of developing a “high-functioning self-cleaning denture” was evaluated under the concept of prevention rather than removal of stains via surface modification (hydrophilic and hydrophobic) using high technologies on the denture surfaces.
Preparation of specimens
Cold-cure denture base resin (PMMA; Palapress Vario, Heraeus Kulzer, Germany) was prepared in accordance with manufacturer’s instructions at a powder/liquid ratio of 7 g / 10 mL, placed in a silicon mold, and polymerized in a pressure pot (Perma Pot UP-III; GC Corp., Tokyo, Japan) for 10 min. Subsequently, a total of 65 specimens were fabricated by sectioning the resins (Type A: diameter, 11.0 mm; thickness, 3 mm) with a low speed precision cutting machine (Isomet, Buehler Ltd., Lake Bluff, IL, USA) (Fig. 1). And a total of 60 specimens were fabricated (Type B: width, 2.0 mm; thickness, 2.0 mm; and length, 25 mm) (Fig. 2). The surfaces of the specimens were polished using a series of #600 to #1500 grit waterproof polishing paper (Sankyo Rikagaku Co., Ltd., Okegawa, Japan). Next, the specimens underwent ultrasonic cleaning (four times) for 8 min each followed by complete removal of the water using Kimwipes. The specimens were then divided into four groups based on the type of surface modification performed as follows: controls (no surface modification); hydrophilic treatment involving ultraviolet (UV) irradiation for 5 min (UV5) and tribochemical coating (TC); and hydrophobic treatment via chemical vapor deposition (CVD).

Fig. 1 Procedure of sample preparation (Type A)
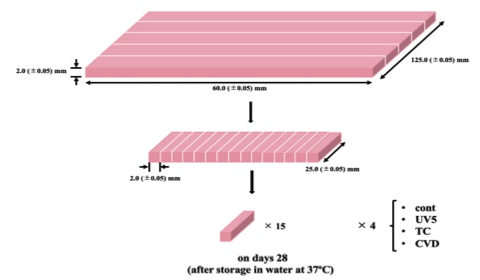
Fig. 2 Procedure of sample preparation (Type B)
Surface modification (hydrophilic treatment)
UV light irradiation in air
The specimens were placed on a wrinkled aluminum foil. A compact UV surface treatment equipment (KOL1-300S, Koto Electric, Tokyo, Japan) was used to apply the UV irradiation (184.9 nm and 253.7 nm) for 5 min (UV5) and 10 min (UV10) each on both sides of the specimens. The distance between the UV lamp and the specimen was 30 mm.
Tribochemical coating (TC)
Rocatec processing (Rocatec, 3M ESPE, Seefeld, Germany) is an airborne particle abrader that injects silica (SiO2) directly on to the surface of the specimen, creating a silicate layer. This process is called friction-chemical surface treatment (TC). In the present study, surface modification was conducted by injecting aluminum oxide particles coated with silica (30 μm in diameter) using a pressure of 0.3 MPa for 15 s from distance of 10 mm from the specimen.
Surface modification (Hydrophobic treatment)
An organic thin film CVD device (UK-S20, N-factory, Nagoya, Japan) was used for this treatment (Fig. 3a). The specimen was placed in a vacuum chamber and air was removed using a vacuum pump (5 Pa). Argon (Ar) gas (20-30 Pa) was injected at high frequency (13.56 MHz; 150-200 W; 1 min) and liquid of tetramethyl orthosilicate (0.5 mL) was added little by little for 1 min (Fig. 3b). Finally, the injection of Ar gas was stopped and the specimen was retrieved after releasing the vacuum.
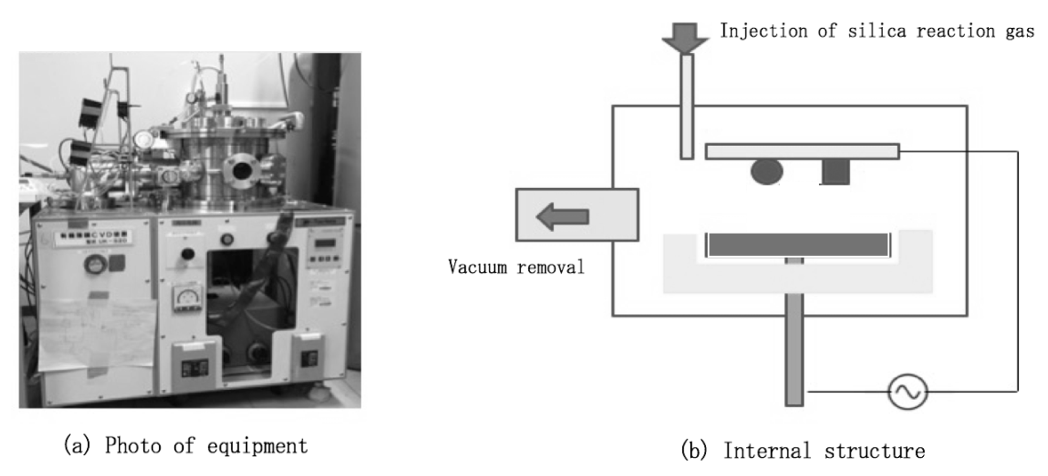
Fig. 3 Process of hydrophobic treatment; (a) Picture showing the equipment used, (b) Internal structure
Surface analysis
X-ray photoelectron spectroscopy (XPS; PHI5000 Versa ProbeII, Ulvac-PHI, Inc., Chigasaki, Japan), Fourier transform infrared spectrometry (FT-IR; ATR PRO450-S, Jasco, Hachioji, Japan) and atomic force microscopy (AFM; SPM-9600, Shimadzu, Kyoto, Japan) were used for surface analysis after modification.
Measurement of surface free energy
Wetting was evaluated using the liquid droplet method with water and di-iodomethane by measuring the contact angle of each treated surface after modification. The average value measured three times was used as a representative value of each specimen, and five specimens of each group were measured (n = 5). Each measurement was conducted using an automatic and dynamic contact angle meter (DCA-VZ, Kyowa Interface Science, Niiza, Japan). Surface free energy was calculated from the values using Owens-Wendt equation [12].
Coloring test
Susceptibility to staining from food was evaluated by mixing 8 g of curry powder (S&B Spicy Curry Powder, S&B Foods Inc., Tokyo, Japan) in 700 mL of warm water (37˚C) and used as a staining solution. Only the bottom half of the specimen was immersed in the solution for 1 min and washed under running water for 10 s. After complete removal of the water, the difference in color (ΔE*ab) between the control (no immersion in curry powder) and experimental groups was measured using a chroma meter (CR-241, Konica Minolta, Inc., Tokyo, Japan). The area not immersed and the area immersed were measured three times each, and the difference between the average values was taken as the color difference representative value of the specimen, and five specimens of each group were measured (n = 5). The following formula was used to measure the color difference based on the L*a*b* color system: ΔE*ab = (Δa2 + Δb2 + ΔL2)1/2.
Three point bending test
For the three point bending test, the resin specimens were was prepared as described earlier, except for a few alterations. The PMMA resin specimens were prepared using the cold cure method according to manufacturer’s instructions (Fig. 2). After four repeats of ultrasonic cleaning and drying, the specimens were divided into four groups as described earlier. The mechanical strength of each specimen was measured using a compact table-top universal testing machine (EZ TEST EZ-S, Shimadzu) at an inter-fulcrum distance of 40 mm and a crosshead speed of 1.0 mm/min. The measurements were performed at on days 28 (after storage in water at 37˚C).
Statistical analysis
One-way analysis of variance was conducted for the data obtained and multiple comparisons were performed using Tukey-Kramer tests ( p < 0.05).
Figures 4 and 5 illustrate the results of the XPS survey spectrum in the control, UV5, and UV10 groups and the TC and CVD groups, respectively. Increase in silica (Si) content was noted at the binding energy position as indicated by an arrow.

Fig. 4 (left) Results of the XPS survey spectra in the untreated (control, red) and atmospheric ultraviolet (UV) irradiation treated (UV5, blue; UV10, green) groups. Arrows indicate areas of no reaction.

Fig. 5 (right) Results of XPS survey spectra in the tribochemical (TC, blue) and (CVD, red) groups. An increase in silica (Si) content was noted at the binding energy position (arrow).
Figure 6 shows the results of the O1s spectrum of the XPS. A decrease in the height of the peak in the high-energy region was observed in the UV5 and UV10 groups relative to the control group.
Figure 7 shows the results of the superposition of the control, UV5, and UV10 groups by FT-IR. In the UV5 and UV10 groups, differences of about 3,400 cm-1 specific to the OH group in the infrared absorption spectrum were noted relative to the control group.
The results of AFM are presented in Fig. 8. Regular micro-asperities (fractal structure) were seen on the surface.
The hydrophilic treatment groups (UV5 and TC groups) showed higher values of surface energy when compared with the control group ( p < 0.05) (Table 1).
Color changes mean and SD in parentheses in the UV5 8.90 (1.18) and TC 5.06 (0.68) groups were significantly greater than that in the control group 2.96 (0.55) ( p < 0.05). However, the CVD group 0.69 (0.95) demonstrated significantly lower changes in color when compared to the controls ( p < 0.05) (Fig. 9).
The CVD group maintained significantly higher values of mechanical strength relative to the control group at on days 28 after storage in water at 37˚C ( p < 0.05) (Table 2).

Fig. 6 (left) Results of the O1s high-resolution spectra using XPS in the control (red) and atmospheric UV irradiated (UV5, blue; UV10, green) groups. A decrease in binding energy peak in the high-energy region was noted in the UV irradiated group when compared with the untreated group.

Fig. 7 (right) Results of superposition of the control (red) and atmospheric UV irradiated (UV5, green; UV10, blue) by FT-IR. A difference in the infrared absorption spectra at around 3,400 cm-1 (characteristic of the OH group) was noted in the UV irradiated group when compared with the untreated group.

Fig. 8 (left) Results of atomic force microscopy (AFM). (a) Control. (b) TC. Formation of fine regular irregular structures (fractal structures) on the surface

Fig. 9 (right) Result of average values of color change after surface modifications (n = 5, p < 0.05)
Table 1 Results of surface free energy measurement
| Surface modifications | Surface free energy (mN/m) |
|---|---|
| Control | 36.1 (3.8) b |
| UV5 | 62.6 (3.0) c |
| TC | 63.0 (4.3) c |
| CVD | 32.6 (6.1) a |
Means and (Standard deviations), Values having the same superscript are not statistically different ( p > 0.05).
Table 2 Results of three point bending test
| Surface modifications | Bending strength (MPa) |
|---|---|
| Control | 88.5 (9.3) b |
| UV5 | 69.6 (7.0) a |
| TC | 88.7 (10.2) b |
| CVD | 99.8 (11.9) c |
Means and (Standard deviations), Values having the same superscript are not statistically different ( p > 0.05).
Active oxygen species (AOS) generated by UV light irradiation in air was used as a hydrophilic surface modification method in the present study. AOS is a collective term used for oxygen species that have high reactivity when compared with oxygen molecules in the ground state (3O2). The production of AOS is simple and can be accomplished by simultaneously applying a specific wavelength of UV light to the ground state oxygen molecules (3O2) or water (H2O). Irradiation of the surface with UV light (wavelength, 185 nm) in air results in the production of ozone by the oxygen molecules. Absorption of wavelengths of 254 nm in the excited state results in the conversion of ozone into active oxygen [13]. The presence of active oxygen atoms on the surface of polymeric molecules due to the strong oxidation reactivity leads to the oxidizing of the surface making it hydrophilic in nature.
XPS was used to evaluate the molecular state of the surface after modification. The binding energy of the electrons and atomic nuclei before release was calculated by measuring the kinetic energy of the core electrons released due to the external photoelectric effect of the X-ray irradiation. The binding energy indicates an element specific value; therefore, XPS measurements can provide both qualitative and quantitative analyses of these elements. Based on the findings of the O1s spectrum (Fig. 6) using XPS, a decrease in the peaks in high-energy regions was observed in the UV irradiated groups when compared with the non-treatment control group. Consequently, it is suggested that UV light irradiation decreased the C-O bonding at a binding energy of approximately 534 eV followed by the formation of a hydrophilic group such as -OH or -COOH [14,15]. In the FT-IR analysis, a difference in the infrared absorption spectrum was noted between the non-treatment group and the UV light irradiation group at around 3,400 cm-1, a spectrum specific to the OH group (Fig. 7). These results indicate that hydrophilicity was achieved by UV light irradiation in air forming chains of OH groups at the molecular ends of the surface. On the other hand, cleavage of the molecular structure of the surface of the denture base resin decreased the mechanical strength, although the effect was limited to the irradiated surface only [16] ( p < 0.05) (Table 2). TC is a surface treatment that results in the formation of a silicate layer that can be silane-treated and may prove useful for adherends that cannot accept silane treatment via conventional methods [17].
Rocatec Pre (particle size, 110 μm) and Rocatec Plus (particle size, 110 μm) have been used for the surface treatments of zirconia and metal in a number of studies [18,19,20,21,22]. However, injection of these materials on to resin surfaces using 0.3 MPa of compressed air may result in excessive roughness or surface damage due to the softness of resins when compared to zirconia or metal. Thus, Rocatec Soft (particle size, 30 μm) was used is the current study [23]. TC forms high concentrations of silica due to the collision of silica particles with the denture base resin and the characteristic regular micro-asperity (fractal structure) on the surface that absorbs water from the air, forming a thin film of water on the surface. This mechanism is considered to increase the hydrophilicity of the entire surface [17,24]. Similarly, hydrophilicity due to increase in free energy on the surface was confirmed without compromising the mechanical strength of the resin, in the present study.
Unlike the environment in the general industry, dentures can be exposed to oil from food in the oral cavity. The self-cleaning effects of the hydrophilic treatments (UV light irradiation in air and TC) in the present study were found to be unsatisfactory after immersion in curry powder.
In the CVD group (hydrophobic treatment), application of high frequency radiation excited and activated the atoms and molecules of gas resulting in the formation of a thin film [25,26]. This method can modify the molecular structure of the surface via a chemical reaction without changing the form of the surface, wherein Si binds to the surface and provides an effective surface treatment [27]. On the other hand, in cases of physical vapor deposition (PVD), which included sputter and vacuum deposition, surface modification is physically achieved by the deposition of particulate raw materials via sputtering or by thermal evaporation [28,29]. However, coverage of miniscule concavities and convexities is challenging; therefore, it is beneficial to use CVD instead of PVD because of its ability to form a uniform and detailed film on the internal and external surfaces of the specimens [30]. Furthermore, the composition and thickness of the film can be adjusted depending on the parts. In addition, as can be seen from the results of XPS (Figs. 4 and 5), increase in Si was observed in the current study indicating that chemical bonding with silica, and the use of hydrophobic treatments resulted in a decrease in surface free energy. The CVD group demonstrated significantly higher values of mechanical strength when compared to the control group after storage for on days 28 after storage in water at 37˚C ( p < 0.05) (Table 2). The mechanical effects of CVD treatment on the denture base resin were negligible because this method did not involve the use of thermal energy. On the contrary, mechanical strength is thought to be increased via chemical cross-linking.
The areas that attract plaque on the denture surfaces are the narrow grooves immediately below the clasps and the inner surfaces of the denture base. These areas are not convex in shape and do not allow for easy polishing of the surfaces. In general, when the prosthetic is inserted in the oral cavity, the surface needs to be polished in order to tolerate the severe conditions in the oral environment. Dentures are polished for three specific reasons as follows: esthetics, so that the shape and surface of the denture resembles the natural anatomy as closely as possible; biological reasons, which include prevention of injury to the oral mucosa and tongue; and chemical reasons, to avoid the accumulation of food debris and denture plaque [23]. Due to the high volume of denture resin in the oral cavity, the level of comfort is largely dependent upon the smoothness of the surfaces. Nevertheless, it is difficult to perform fine surface finishing in certain small areas such as the interdental papilla due to limited access. The hydrophobic surface modifications used in the current study were able to lower water absorption by the denture resin and contributed to improvements in the self-cleaning of areas that are hard to reach. The results of the present study indicate that plasma CVD treatment of PMMA resin is an effective denture surface modification method that adds the characteristic of stain resistance to commercially available denture base materials.
Acknowledgment
This work was supported by JSPS KAKENHI Grant Number JP18K09670.
Conflict of Interest
None