2019 Volume 19 Issue 2 Pages 51-57
2019 Volume 19 Issue 2 Pages 51-57
Purpose: This study compared the ability of three methyl methacrylate (MMA)-p-styrene sulfonic acid copolymer (MS polymer)-based desensitizers (Pain-Free Gel Dentin Desensitizer; PF-Gel, No-Mix Pain-Free Desensitizer; PF, No-Mix Pain-Free F Desensitizer; PF-F) to occlude dentinal tubules. Materials and Methods: Dentin disc specimens were prepared from bovine incisors and the desensitizers were applied. After dehydration for 24 h or immersion for 1 week in artificial saliva, the surfaces were observed by scanning electron microscope (SEM). PF-Gel specimens also underwent SEM/energy dispersive X-ray spectroscopy (EDS). Results: Application of the desensitizers produced complete occlusion of the dentinal tubules. Most dentinal tubules re-opened after immersion for 1 week in artificial saliva (PF and PF-F samples), but in PF-Gel samples, the dentinal tubules remained occluded. SEM/EDS analysis demonstrated that the granular crystals adhering to the dentin surface contained oxygen, carbon, and calcium, and the plugs occluding the dentinal tubules contained calcium and phosphorus. Conclusion: The findings in this study confirm the sustainable effect of PF-Gel on the occlusion of dentinal tubules.
Cervical dentin hypersensitivity, the most common cause of reversible dental pulpitis, is a common symptomatic condition that causes discomfort in adult patients [1]. The clinical symptoms of cervical dentin hypersensitivity are principally caused by exposure of dentinal tubules as a result of enamel loss and/or gingival root surface exposure caused by gingival recession, abrasion, attrition, erosion, and abfraction [2,3]. Exposure of dentin with patent dentinal tubules results in activation of A-delta pulpal nerve fiber endings, causing pain [4]. Several hypotheses have been proposed to explain the mechanism of cervical dentin hypersensitivity. The hydrodynamic theory proposed by Brännström [5,6] is the most generally accepted [7]. This theory suggests that pain-provoking stimuli increase the movement of dentinal tubular fluid or change the flow direction, stimulating A-delta pulpal nerve fibers around odontoblasts, and thereby leading to cervical dentin hypersensitivity.
Three main strategies have been introduced to treat cervical dentin hypersensitivity: 1) depolarization of the nerve by topical application of potassium nitrate (KNO3) [8]; 2) biological fixation of dentinal fluid by denaturing protein using glutaraldehyde-containing desensitizer [9,10]; and 3) physical occlusion of the exposed dentinal tubules [11]. Oxalate-containing products have been tested both in vitro [12,13,14] and in vivo [15,16].
Methyl methacrylate (MMA)- p -styrene sulfonic acid copolymer (MS polymer) is a water-soluble copolymer. MS polymer cross-links with Ca2+ released from the tooth substrate and is immobilized on the tooth surface. MS polymer-based desensitizers have been reported to seal the dentinal tubules and reduce hypersensitivity from thermal, mechanical and chemical stimulation [17,18]. However, an MS polymer-based desensitizer containing 1% oxalic acid (No-Mix Pain-Free, Sun Medical, Moriyama, Japan) has been reported to have lower tubule occluding ability than a fluoro alumino calcium silicate-based desensitizer (Nanoseal, Nippon Shika Yakuhin, Shimonoseki, Japan) and a calcium phosphate-based desensitizer (Teethmate Desensitizer, Kuraray Noritake Dental, Tokyo, Japan) [13].
Recently, a modified MS polymer-based desensitizing agent was developed with the addition of a thickener to convert it into gel form, with the aim of improving retention on the tooth surface. Strengthening of the dentin and reduction of intra-dental nerve excitability could also be expected as a result of the addition of sodium fluoride and potassium phosphate [18,19]. However, these effects have not yet been verified. The aim of this in vitro study was therefore to investigate whether this newly developed MS polymer-based desensitizing gel could increase the occlusion of the dentinal tubules. Also, changes in the tooth surface after immersion in artificial saliva for 1 week were evaluated to investigate the sustainability of the dentinal tubule seal after application of the MS polymer-based desensitizing gel.
Three MS polymer-based dentin desensitizers were used in this study; Pain-Free Gel Dentin Desensitizer (Sun Medical), No-Mix Pain-Free Desensitizer (Sun Medical), and No-Mix Pain-Free F Desensitizer (Sun Medical) (Table 1).
Table 1 Materials used
| Product (Manufacturer) | Ingredients | pH | Lot No. | Code |
|---|---|---|---|---|
| Pain-Free Gel Dentin Desensitizer* (Sun Medical, Moriyama, Japan) | MS polymer, 1% oxalic acid, water, sodium fluoride (900 ppm F−), acidity regulator (potassium phosphate salt), thickener, others | 2 | LR11 | PF-Gel |
| No-Mix Pain-Free** (Sun Medical) | MS polymer, 1% oxalic acid, water | 1.5 | LL1 | PF |
| No-Mix Pain-Free F Desensitizer*** (Sun Medical) | MS polymer, 1% oxalic acid, water, sodium fluoride (3,000 ppm F–) | 2 | MK1 | PF-F |
MS polymer, methyl methacrylate (MMA)- p -styrene sulfonic acid copolymer
*MS Coat Hys Block Gel, in Japan
**MS Coat ONE, in Japan
***MS Coat F, in Japan
Specimen preparation
Twelve extracted and non-damaged bovine incisors, frozen to maintain freshness, were defrosted, and the adherent soft tissue was removed under running tap water. They were cut at the enamel-dentin junction using a low-speed diamond saw (Isomet, Buehler, Lake Bluff, IL, USA) and the pulp and soft tissue were removed. The labial side of the enamel surface was abraded under a stream of water with #180 SiC paper to expose a flat dentin surface. The specimens were sequentially abraded under a stream of water with #600 and #1,200 SiC paper to form a 10 × 10 × 1 mm disc-shaped dentin specimen. The labial surface of the dentin specimens was brushed for 3 min using a manual toothbrush (Butler #411, Sunstar Suisse S.A., Etoy, Switzerland) and commercial dentifrice (White & White, Lion, Tokyo, Japan), and ultrasonically cleaned in ultra-pure water for 35 min. Thereafter, these dentin specimens were sterilized in an autoclave (BS-245, Tomy, Tokyo, Japan) at 121˚C for 20 min.
Morphological analysis of the desensitizer-applied dentinSeven dentin discs were divided into four groups (2 discs for PF-Gel, 2 discs for PF, 2 discs for PF-F, and 1 disc for non-applied control) for morphological analysis of the dentin. The discs were subjected to one of the following treatments: PF-Gel, A rice grain-size amount of PF-Gel was applied to the dentin surface for 30 s, and thoroughly rinsed with running tap water; PF, One drop of PF was applied to the dentin surface for 120 s without rubbing and dried with an air syringe; and PF-F, One drop of PF-F was applied to the dentin surface for 120 s without rubbing and dried with an air syringe. The remaining dentin disc was used as a control.
The conditioned specimens were rinsed with running tap water and dried with air pressure. After dehydration for 24 h in a desiccator at room temperature, one specimen from each group was placed on an aluminum stub and Pt-coated using a super fine sputter coater (ESC-101, Elionix, Hachioji, Japan) for 120 s and examined under scanning electron microscopy (SEM) (LSM-5610LV, JEOL, Akishima, Japan) at 3,500× magnification. The other specimens were fractured longitudinally at the center with a cutting plier. They were placed on a stub and Pt-coated, and the cross-sectioned surface was examined under SEM at 8,000× magnification.
SEM analysis after 1-week immersion in artificial salivaThree dentin discs were coated with PF-Gel, PF, or PF-F as for the morphological analysis of the dentin. The specimens were immersed in 50 mL of artificial saliva (1.09 mmol/L CaCl2, 0.68 mmol/L KH2PO4, 30 mmol/L KCl, 2.6 µmol/L NaF, 50 mmol/L HEPES; pH 7.0) at 37˚C for 7 days [20]. The immersing solution was changed every 24 h. Each specimen was then rinsed with ultra-pure water and dried with air pressure. After dehydration for 24 h in a desiccator at room temperature, they were placed on an aluminum stub, Pt-coated and examined under SEM.
Energy dispersive X-ray spectroscopy (EDS) analyses of PF-Gel specimensThe remained two dentin discs were coated with PF-Gel, and one was immersed in 50 mL of artificial saliva in the same manner (37˚C for 7 days). These specimens were cleaned, air-dried, and dehydrated for 24 h. Each specimen was osmium-coated for 10 s (7 Pa of vacuum, 10 mA) using osmium coater (Neoc-STB, Meiwafosis, Tokyo, Japan) and analyzed at a decomposition depth of about 100 nm under high-resolution SEM/EDS (S-4800, Hitachi High-Technologies, Tokyo, Japan / Quantex Flat Quad, Bruker, Yokohama, Japan) at 6 kV.
Figure 1 demonstrates SEM images of the dentin surface and longitudinal cross-section in each group that did not undergo the 1-week immersion in artificial saliva. The control specimen (not treated with any desensitizer) confirmed the absence of a smear layer on the dentin surface because scratches caused by abrasion with the SiC paper could be clearly detected (Fig. 1d, black arrow). Open dentinal tubules were all clearly visible (Fig. 1d). In contrast, complete occlusion of the dentinal tubules was seen after treatment with any of the MS polymer-based desensitizing agents (Fig. 1a-c). Globular-like deposits could also be detected on the dentin surface and within the dentinal tubules. Sagittal views showed that the dentin surface was completely covered with a thin layer of desensitizer which appeared to completely occlude the dentinal tubules in all PF-Gel, PF and PF-F specimens (Fig. 1e-g).
Figure 2 shows SEM images of the dentin surfaces in each group after a 1-week immersion in artificial saliva. PF-Gel specimens were still covered with globular-like deposits on the dentin surface and within the dentinal tubules, and no open dentinal tubules were detected (Fig. 2a). However, almost all dentinal tubules were open in PF and PF-F specimens (Fig. 2a,b).
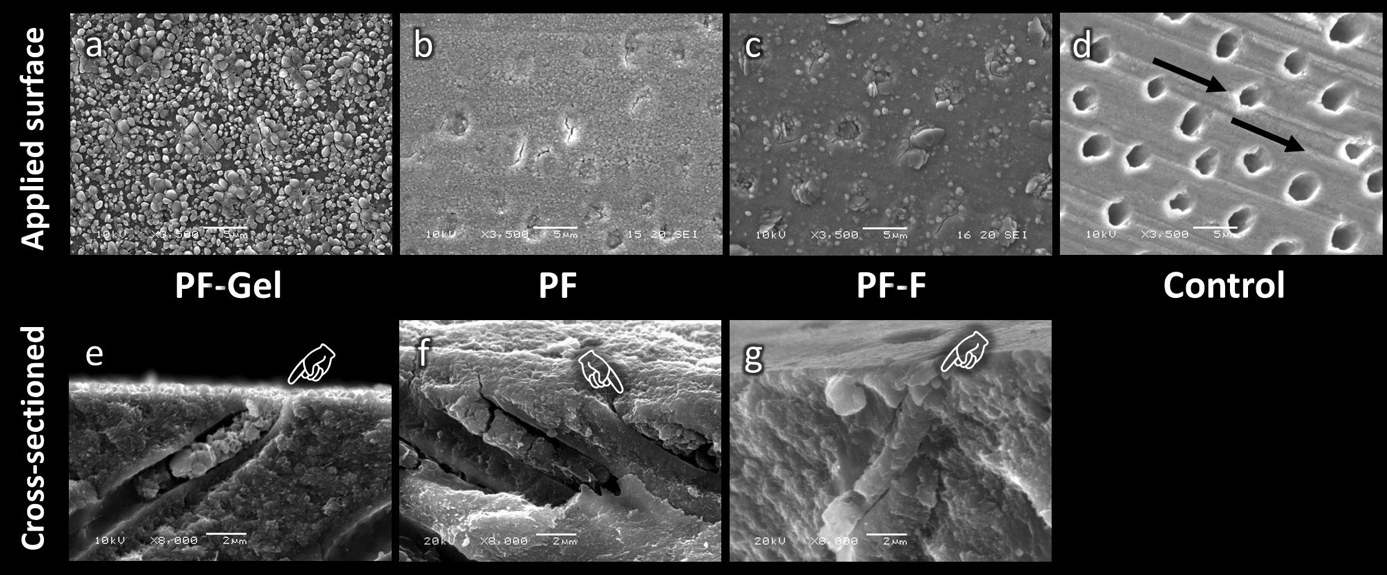
Fig. 1 Scanning electron micrographs showing the dentin surface (original magnification 3,500x) and longitudinal fractures (original magnification 8,000x) after application of MS polymer-based desensitizers; PF-Gel (a, e), PF (b, f), PF-F (c, g), and the control dentin surface (no desensitizer applied) (d). Globular-like deposits can be seen on the treated dentin surface and within the dentinal tubules. The dentin surface is completely covered with a thin layer of desensitizer which appears to completely occlude the dentinal tubules (pointers). The control dentin surface shows scratches caused by abrasion with SiC paper (black arrow), and the dentinal tubules are all open. Bar scale 2 µm.
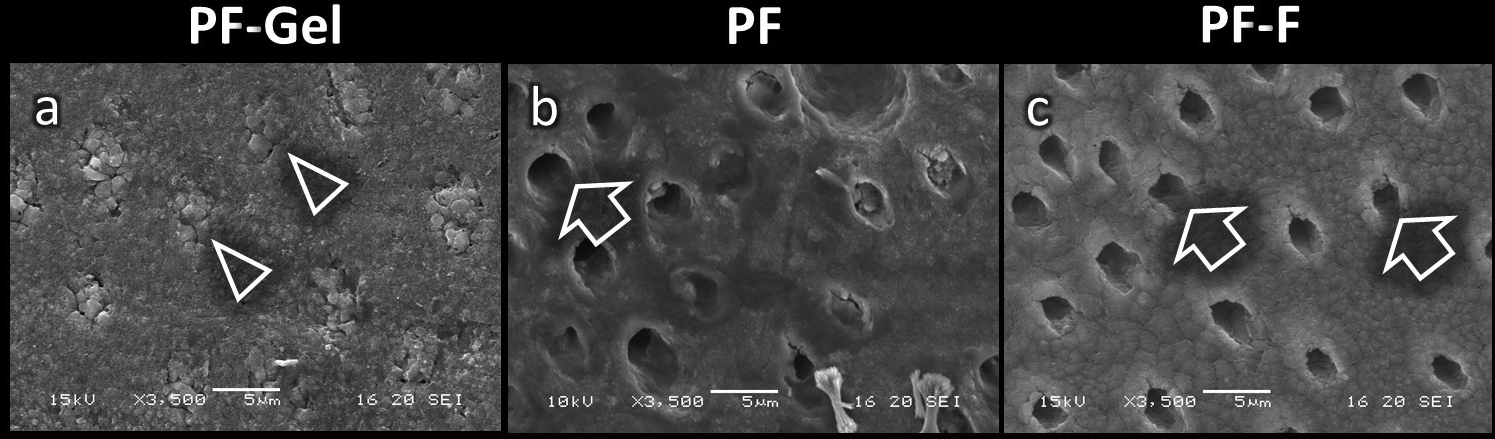
Fig. 2 Scanning electron micrographs of the dentin surface after application of MS polymer-based desensitizers followed by immersion for 1 week in artificial saliva (original magnification 3,500x); PF-Gel (a), PF (b), and PF-F (c). Some dentinal tubules are open in PF and PF-F specimens (open arrows). However, dentinal tubules remain sealed in the PF-Gel specimens after immersion for 1 week in artificial saliva (white arrowheads). Bar scale 5 µm.
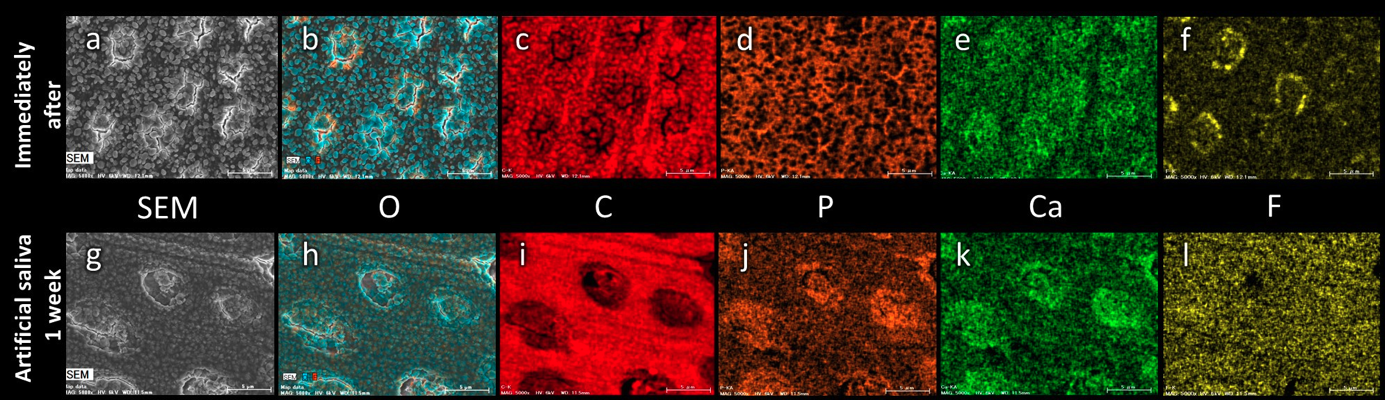
Fig. 3 Scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDS) mapping images of the dentin surfaces of PF-Gel specimens (original magnification 5,000x). After application of PF-Gel, granular crystals can be seen adhering to the dentin surface, and oxygen (O), carbon (C) and calcium (Ca) are present. The plugs of the dentinal tubules show the presence of Ca and phosphorus (P). Fluorine (F) can be detected locally around the dentinal tubules. After immersion in artificial saliva for 1 week, granular deposits can be seen on the dentin surface. Although Ca and O have diminished around the intertubular dentin, C is retained. The plugs of the dentinal tubules show the presence of Ca and P, and F can be detected over the entire dentin surface. Bar scale 5 µm.
EDS analyses of PF-Gel specimensFigure 3 shows the mapped surfaces of the dentin coated with PF-Gel under SEM/EDS analysis. The specimens examined immediately after coating showed granular crystals containing oxygen, carbon and calcium adhering to the dentin surface (Fig. 3b,c,e). The plugs in the dentinal tubules showed the presence of calcium and phosphorus (Fig. 3d,e). Fluorine was detected around the dentinal tubules (Fig. 3f). The specimens immersed for 1 week in artificial saliva exhibited smaller and fewer granular deposits on the dentin surface. The plugs in the dentinal tubules showed the presence of calcium and phosphorus (Fig. 3j,k). Fluorine was detected over the entire dentin surface (Fig. 3l).
PF has a relatively strong demineralization effect because of its strong acidity (pH 1.5). When MS polymer is applied to the dentin surface, it can react with Ca2+ ions supplied by demineralized hydroxyapatite from the smear layer, producing an MS polymer-Ca complex and covering the exposed dentin surface. Soluble oxalate salts have been known to form and deposit slightly soluble calcium oxalate (CaC2O4) crystals on the dentin surface by binding with Ca2+ ions from the smear layer [21,22,23]. Therefore, it was thought that application of oxalic acid-containing MS polymer-based desensitizer would result in occlusion of the dentinal tubules by the MS polymer-Ca complex and CaC2O4 crystals, thereby reducing the permeability of the dentinal tubules, and contributing to the prevention of dentin hypersensitivity [16]. The present findings confirming that the dentinal tubules are completely occluded immediately after applying PF support previous studies that demonstrated an immediate reduction in dentin permeability after application of an oxalic acid-containing MS polymer-based desensitizer [14,24].
PF-F contains sodium fluoride in the aqueous solution. Although fluoride ions do not contribute directly to tubular occlusion, acidic fluoride varnishes have been reported to react with dentin to release Ca2+ that can form calcium fluoride (CaF2) with the varnish base; these CaF2 particles can migrate into and occlude the dentinal tubules [25]. Oshima et al. recently reported that PF-F-treated dentin showed significantly lower mineral loss than PF-treated dentin after acidic challenge (5 h, pH 4.5) [18]. They also observed a 2-3 µm-thick layer-like structure covering the dentin surface and the dentinal tubules even after the acidic challenge. Therefore, PF-F is expected to not only prevent dentin hypersensitivity, but also prevent root dentin demineralization.
The results of our study and other previous studies showed that oxalate salts are effective in producing calcium oxalate crystals on the dentin surface. However, calcium oxalate crystals have been reported to either partially dissolve in saliva or oral fluids, or disappear during toothbrushing [15,20,23,26,27]. Therefore, the sustainability of the MS polymer-based desensitizing agents on the occlusion of dentinal tubules after immersion in artificial saliva also was evaluated. The present results support the previous reports [27] that found that calcium oxalate crystals dissolved on the dentin surface and the dentinal tubules were open in both PF and PF-F specimens. Therefore, in the clinical situation, it is recommended to apply them repeatedly at each visit.
Although the PF-Gel-coated dentin surface was covered with a granular precipitate in the same way as the PF and PF-F specimens, the granules were larger and denser than those of the PF and PF-F specimens. Because SEM/EDS evaluation detected that the granular crystals were composed of O, C, and Ca, it was presumed that they were crystals of calcium oxalate. Additionally, the localization of phosphorus and calcium observed around the dentinal tubules suggests the formation of calcium phosphate or its fluoride (calcium fluoride). Therefore, thickeners added to the PF-Gel may have helped to retain the MS polymer and oxalic acid on the tooth surface [28], thereby efficiently promoting the formation of calcium oxalate, calcium phosphate, and calcium fluoride crystals.
In contrast with the PF and PF-F specimens, the dentinal tubules of the PF-Gel specimens remained completely closed even after a 1-week immersion in artificial saliva. Additionally, the granular crystals remained on the dentin surface, although they were smaller than those in the immediate specimen. Varoni et al. recently demonstrated the effect of a novel potassium oxalate hydrogel in reducing the solubility of potassium oxalate crystals, and thereby improving the efficacy of potassium oxalate as a dentinal tubule-occluding agent [29]. They presumed that the time-dependent occlusive effect on dentinal tubules is probably a result of the presence of the active ingredient, rather than a direct effect of the hydrogel itself. Our results supported their presumption. Therefore, the thickeners in PF-Gel may also have reduced the solubility of the calcium oxalate crystals and allowed the resin-based component (MS polymer) to be retained.
Localized calcium and phosphorus were also detected at the plugged dentinal tubules, and deposits of fluorine were detected over the entire dentin surface after immersion for 1 week in artificial saliva. Therefore, the crystals occluding the dentinal tubules could be calcium phosphate and calcium fluorophosphates Ca5(PO4)3F. Formation of these crystals may not only suppress the penetration into the dentinal tubule, but may also inhibit the demineralization of exposed root dentin in the same way as PF-F [18]. PF-Gel contains potassium phosphate as a pH adjuster, and this component may also contribute a nerve-numbing effect caused by the potassium ions [8,29].
Acknowledgments
This work was partially supported by JSPS KAKENHI Grant Numbers JP 17K11715 and JP 16K20464.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.