2019 Volume 19 Issue 2 Pages 65-69
2019 Volume 19 Issue 2 Pages 65-69
Purpose: Auto-polymerized poly(methyl methacrylate) (PMMA) resin cannot be bonded to polyamide thermoplastic resin. This study evaluated the bonding strengths of auto-polymerized PMMA resin to nylon denture bases using adhesive agents. Materials and Methods: The surfaces of polyamide thermoplastic resin plates were polished using abrasive paper and blasted with 50 μm alumina particles. An adhesive agent, 4-META/MMA-TBB resin (Super-Bond) was used on the surfaces under the following conditions: monomer + catalyst (4:1, Group 1), monomer + catalyst (8:1, Group 2), monomer + catalyst (4:1) + polymer (Group 3), monomer + catalyst (8:1) + polymer (Group 4), treatment with dichloromethane (Group 5), alumina-blasted (Group 6), without treatment (Group 7), and PMMA resin (Group 8). Auto-polymerized PMMA resin was poured into a Teflon ring and polymerized according to the manufacturer’s instructions. After polymerization, the specimens were stored in 37˚C distilled water for 24 hours. The specimens for each condition were thermo-cycled for 5,000 cycles. The tensile bond strengths were measured on an autograph at a crosshead speed of 2.0 mm/min. The data obtained (n = 13) were analyzed using a two-way ANOVA and Tukey’s multiple comparisons test (α = 0.05). Results: The bonding strengths of Groups 1 to 4 were significantly higher than for Groups 5 to 7. No significant differences were found among Groups 1-4, although Group 3 had the highest bonding strengths. Conclusion: Auto-polymerized PMMA resin will adhere to polyamide thermoplastic resin using 4-META/ MMA-TBB after alumina-blasting.
Several thermoplastic resins, such as polyamide, polyethylene terephthalate, polycarbonate, and acrylic resins, have been developed and are commercially available for flexible denture construction [1,2,3,4,5,6]. In particular, polyamide (nylon) thermoplastic resin has the highest elasticity for flexible resin clasps and is more commonly used than the other thermoplastic resins [7,8,9]. However, there are several disadvantages of the clinical use of nylon dentures, for instance, poor machinability, i.e., cutting and grinding, difficulty of polishing, poor adjustability of the retentive force of the resin clasp, easy to roughen on the surface, and little bonding ability of the auto-polymerized poly(methyl methacrylate) (PMMA) resin [2,4]. Therefore, it is difficult to achieve the chair-side repair of the flexible dentures using auto-polymerized PMMA resin due to denture breakage, detachment of the artificial teeth, difficulty of adding the artificial teeth if a tooth is missing, direct relining, and so on.
Using 4-META/MMA-TBB resin as the adhesive agent, chair-side relining or denture repair of polyamide dentures with auto-polymerized PMMA resin can be developed. The aim of this study was to evaluate the bonding strengths of auto-polymerized PMMA resin to polyamide denture polymer using adhesive agents.
Rectangular polyamide thermoplastic resin plates (Bioplast, Denken-Highdental, Kyoto, Japan; Lot, 1303217) (10.0 × 10.0 × 2.0 mm) were fabricated using the injection molding technique (injection pressure 1.5 MPa) according to the manufacturer’s instructions (MIS-II, i-CAST, Kyoto, Japan). After deflasking, all the surfaces of the resin plates were finished and polished using abrasive paper (600 grit) and blasted with 50 µm alumina particles (0.48 MPa). As an adhesive agent, 4-META/MMA-TBB resin (Super-Bond, Sun Medical, Moriyama, Japan) was applied on the bonding surfaces using following four mixing conditions: monomer + catalyst (4:1, Group 1), monomer + catalyst (8:1, Group 2), monomer + catalyst (4:1) + polymer (Group 3), and monomer + catalyst (8:1) + polymer (Group 4). Groups 1-4 were alumina-blasted before application of adhesive agent (Table 1). Following three surface treatment conditions were added to compare their use with 4-META/MMA-TBB resin: treatment with dichloromethane (Meta base M resin primer, Sun Medical; Group 5), alumina-blasted (50 µm Al2O3; Group 6), without surface treatment (Group 7), and PMMA resin without surface treatment (Group 8). As a control, heat-polymerized PMMA plates (Acron, GC, Tokyo, Japan) were also prepared for comparison between the conventional dentures and the nylon dentures. After application of the adhesive agent, auto-polymerized PMMA resin (Meta base M, Sun Medical) was poured into a Teflon ring (3.0 mm diameter, 1.0 mm height) on the bonding surface using the brush-on technique and polymerized according to the manufacturer’s instructions (Fig. 1). After polymerization, all the specimens were stored in distilled water at 37˚C for 24 hours before tensile testing. Thermo-cycled (5,000 cycles; 5˚C, 1 min-55˚C, 1 min) specimens (TC5000) were also prepared for each condition.
Table 1 Surface treatments
| Group | Surface treatment | Adherend |
|---|---|---|
| 1 | Super-Bond Monomer + Catalyst (4:1) | Polyamide |
| 2 | Super-Bond Monomer + Catalyst (8:1) | Polyamide |
| 3 | Super-Bond Monomer + Catalyst (4:1) + Polymer (1/2 cup) | Polyamide |
| 4 | Super-Bond Monomer + Catalyst (8:1) + Polymer (1/2 cup) | Polyamide |
| 5 | Dichloromethane | Polyamide |
| 6 | Alumina blasting | Polyamide |
| 7 | Without surface treatment | Polyamide |
| 8 | Without surface treatment (control) | PMMA |

Fig. 1 Bonded specimen

Fig. 2 Assembly for tensile bond strength testing
Each specimen was mounted on a screw-driven mechanical testing machine (AG-IS, Shimadzu, Kyoto, Japan). The bond strengths were measured at a crosshead speed of 2.0 mm/min (Fig. 2). The data obtained (n = 5) were analyzed by two-way ANOVA and Tukey’s multiple comparisons test at α = 0.05.
The abraded and sandblasted surfaces of the thermoplastic resin were observed with a scanning electron microscope (SEM, ×500 magnification, JSM 6300, JEOL, Akishima, Japan) with an accelerating voltage of 15 kV.
The tensile bond strengths of all conditions are indicated in Fig. 3. The specimens in Groups 1 to 4 showed significantly higher bonding strengths than Groups 5 to 7 ( p < 0.05) and were comparable to heat-polymerized PMMA at TC0. Of the 4-META/MMA-TBB resin, Group 3 had the highest bonding strengths ( p > 0.05) followed by Groups 2, 4, and 1. After thermo-cycling, the bonding strengths of all the specimens significantly decreased ( p < 0.05). In particular, all the Group 6 and 7 specimens failed before testing. Comparing heat-polymerized PMMA, Groups 1 to 4 decreased approximately one second of bonding durability.

Fig. 3 Bond strengths of PMMA resin to polyamide thermoplastic resin
Identical letters indicate that the values are not statistically different ( p > 0.05).
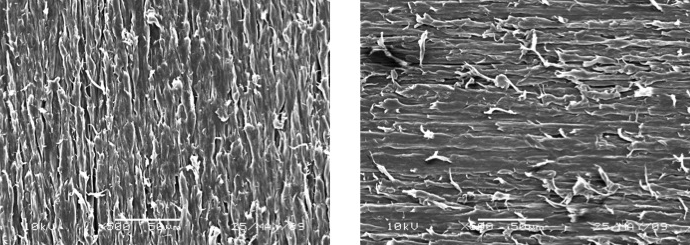
Fig. 4 Scanning electron micrographs of polyamide: left, alumina-blasted and polished; right, polished
SEM observationElectron micrographs of the abraded and alumina-blasted surfaces of the polyamide plates are shown in Fig. 4. The surfaces abraded using abrasive paper (#600) were rougher and had many sharp edges. Although the alumina-blasted surfaces were rougher than the abraded surfaces, few sharp-edged surfaces were observed.
Clinically, fractures of nylon dentures have been rarely observed because of their mechanical characteristics. However, polyamide is noted fatal problem, namely, it cannot be bonded to PMMA for denture repair or reline. Thus, it must develop the adhesion method of polyamide and PMMA in chair side repair.
Because polyamide is chemically remarkable stable, dichloromethane as denture primer for showed significantly lower bonding strengths than the other treatment [10]. The 4-META/MMA-TBB resin has found sound evidence that adhesion between dentin to metal prosthesis as a resin cement [11]. In this study, 4-META/MMA-TBB was applied as adhesive agent of the bonding of polyamide thermoplastic resin and PMMA. Specimens with only 4-META/MMA-TBB monomer + catalyst as surface treatment showed lower bonding strength compared with monomer + catalyst with slight polymer. Condition of monomer + catalyst with slight polymer indicated similar bonding strength to acrylic denture base resin (control) without thermo-cycling. Thus, mixing polymer is absolutely necessary for adhesion between 4-META/MMA-TBB resin and auto-polymerized acrylic resin.
This bonding mechanism would be occurred as minute mechanical binding occurred by sandblasting treatment. It must be difficult that invaded to the molecular gap of polyamide because the molecular weight of mixed 4-META/MMA-TBB resin was 239×103. The surface property greatly affects to adhesive strengths [12,13,14,15]. Minami et al. [16] examined the bonding strength of the acrylic denture base resin for repair. MMA monomer can invade by dissolving the acrylic resin surface using dichloromethane. Ohkubo et al. [17] described the minute mechanical surface treatment using the alumina-blast was needed to make adhere of acrylic resin minute to framework alloy which MMA could not invade. Polyamide resin has a chemical superior homeostasis, the surface cannot be dissolved with menstruum by an MMA monomer or the dichloromethane similar to metal. Therefore, the minute mechanical bond is also necessary to obtain high bonding strengths of PMMA to thermoplastic resin. Many sharp scratches were observed on the surface of polyamide by abrasive paper (#600) from the SEM image (Fig. 3). However, the mechanical retention for higher adhesion was occurred by countless unevenness on the polyamide treated by blasting in this study.
Although many studies were reported on the bonding of auto-polymerizing resin to conventional denture base resins, there were few researches on the polyamide denture polymers. Katsumata et al. [10] reported that the shear bond strengths to another polyamide polymer (Lucitone FRS, Dentsply) were significantly greater using the Rocatec System. This procedure applied the silane coupling treatment after silicate coating on the bonding surface. However, an easier procedure without any special equipment is desirable for chair-side repair. Nevertheless the bonding durability was only half that of conventional PMMA denture base resin, the repair or reline for polyamide denture could be easily accomplished at chair-side using 4-META/MMA-TBB resin. Another reason why, as a result, the abrasion from margin of bonding materials is occurred, increased quantities of PMMA as bonding material make curing shrinkage is increase [18] even if PMMA and polyamide thermoplastic resin cannot adhere chemically. Therefore, the use of slight 4META-MMA/TBB as adhesive agent, not only it is hard to exfoliate from polyamide thermoplastic resin as adherend by curing shrinkage is decrease but also it can be adhered to PMMA.
After thermo-cycling (5,000 cycles), the bonding strengths of all conditions were showed significantly decreased except the control. This result also suggested that the long-term stability of the bond might be slightly prevented by mixing polymer to monomer + catalyst. This study could not elucidate the adhesion mechanism between polyamide and 4-META/MMA-TBB. Further study is necessary to examine the effect of the ingredient of 4-META/MMA-TBB gives to the surface of the polyamide.
Conflicts of Interest
There are no conflicts of interest.