2017 Volume 125 Issue 1 Pages 15-24
2017 Volume 125 Issue 1 Pages 15-24
Spread by infected galleys coming from Kaffa (Crimea), the Black Death reached Genoa, as it now seems, in the late summer of 1347 AD. Genoa functioned as an epicentre from which the contagion was spread into the mainland through a complex system of routes, which linked Liguria to northern and central Italy. Along these routes various institutions were found, namely ‘ospitali’ (hospitals) and ‘stationes’ (stations), where traders and pilgrims stopped to rest and recuperate. In 2006 a multiple burial archaeologically dated to the second half/end of the 14th century was discovered in the cemetery pertaining to the ‘ospitale’ of San Nicolao (Genoa). The excavation showed that it contained the remains of four individuals: a 38–40 week pregnant woman with her fetus and two sub-adults. Stratigraphy showed that these individuals were buried simultaneously. Given that the dating of the burial fits the arrival of the Second Pandemic in Europe, it was hypothesized that they might have died during the Black Death epidemic. The identification of Yersinia pestis F1 antigen in three of four individuals corroborated this hypothesis. Here we report the first evidence of Y. pestis infection in 14th-century Liguria and discuss the possible mechanisms of plague dissemination from Genoa into the surrounding regions. In fact, the ‘ospitale’ of San Nicolao, located at 792 m a.s.l. into the Bracco Massif, was used as a resting place/hostel by traders and travellers (e.g. pilgrims heading for Rome). This ‘ospitale’ represented a key point leading into a system of pathways forming the initial part of the Vie Romee better known under the name of Via Francigena in the Italian territory and, as a consequence, was the ideal site from which plague could be disseminated.
The arrival and spread of the Black Death in Italy was truly a momentous, albeit tragic, event in European history. These events were closely associated with the fact that, at the time, Italian cities, especially Genoa and Venice, were leading commercial sea powers, which sent their huge galleys all the way to the Crimea, Egypt, around the Mediterranean Sea, through Gibraltar and all the way to Bruges and London. These enormous voyages were supported by a network of harbours, trading stations and factories along the sea lanes (Pounds, 1974; Benedictow, 2013).
Italian cities were also leading proto-industrial centres. From central and northern Italy, huge quantities of goods were transported also by land along tracks and roads across Austria and Switzerland into France, Germany, Bohemia and Hungary (Pounds, 1974; Cipolla, 1976; Lopez, 1976).
From the end of thirteenth century, Italy was increasingly overpopulated in the sense that, under the prevailing agricultural structures and technologies inland, production of grain and other foods did not suffice to feed the population of around 12.5 million (Pinto, 1996). A large-scale system of importation and distribution of food, especially cereals— because of their high calorific value in relation to weight and transport costs—from Sicily, Provence and Egypt, was developed by Italian cities or city states, organized by socalled Offices of Abundance. In addition, there were private importers and small-scale distributors of grain and flour who met demand in the countryside and local small towns (Cipolla, 1976; Pinto, 1985, 1996). In an overpopulated Europe in relation to means of food production, huge volumes of cereals were imported to commercial hubs on the coasts and spread by land and river transport across the continent.
From the late 1200s, Europe was becoming increasingly highly vulnerable to importation of plague. The dynamic spread of the disease was facilitated by a wide-flung and fine-meshed network of trade of grain and flour, highly suitable for the efficient dissemination of infective rat fleas. The spread of the Black Death all over Europe shows the characteristic epidemiological pattern and defining features of rat-and-rat-flea-borne plague (Benedictow, 2010, 2017). In fact, plague develops according to a quite strict epidemiological pattern. General epidemiological knowledge on plague allows the identification of a number of phases occurring between the introduction of contagion in a given community (i.e. village, town, city, metropolis) and the manifestation of the epidemic. Three main phases can be identified. Phase 1 corresponds to the dissemination of plague among rats and includes the enzootic and epizootic steps. Phase 2 is the endemic phase during which the first cases of human plague manifest and are usually concentrated in a few houses; this phase also includes the incubation period, i.e. the time elapsing from the infective bite to when the person falls ill and the average duration of disease in fatal cases. Phase 3 corresponds to the development of the proper epidemic (Benedictow, 2017). A vast amount of knowledge has allowed plague scholars to trace a time horizon of the epidemiological phases: 10–14 days (on average 12 days for the epizootic to manifest) + 3 days (fasting fleas that have lost their natural hosts, the rats, and are unable to feed on them turn to humans) (Pollitzer, 1954) + 0.5–1 days (the time assumed to be required before the first infective rat fleas find the opportunity to feed on humans giving rise to the first infective transmission) + 7 days (endemic) + 8 days (incubation and illness for the last endemic cases) + 8 days (incubation and illness for early epidemic cases infected during the endemic phase)—in all 39 days or about 5–6 weeks (Benedictow, 2017). Depending on the size of the community, the period between the time of contagion and the recognition of the presence of the Black Death varied from about 5.5–6 weeks in small villages and townships, to 6–7 weeks in towns, to 7 weeks in cities and quite likely a week longer in the few metropolises of the time with about or over 100000 inhabitants (Benedictow, 2017). Seasonality is also an important factor to be considered. Winter weather tends to slow down or stop the epidemiological processes. This implies that often the epidemic activity disappears and the presence of plague will revert to a mainly epizootic phase caused by a rapid depletion of the number of fleas (Benedictow, 1996), a strong diminution of bacteraemia in infected rats, a slower reproduction rate of bacteria in the fleas’ stomach system and, consequently, a much less frequent development of blockage in fleas. The slowdown in areas with cool winters, such as France, or a temporary seasonal break in areas with cold winters, such as northern Germany and the Nordic countries, could be shown by plague historians (Benedictow, 2017).
With the Italians’ wide-ranging commercial contacts and large-scale cereal importation the chances were substantial and increasing that plague would at some point be transported by Italian galleys, from regions with rodent plague reservoirs, into the European economic and commercial powerhouse of Italy with maximum disseminative potential (Figure 1).
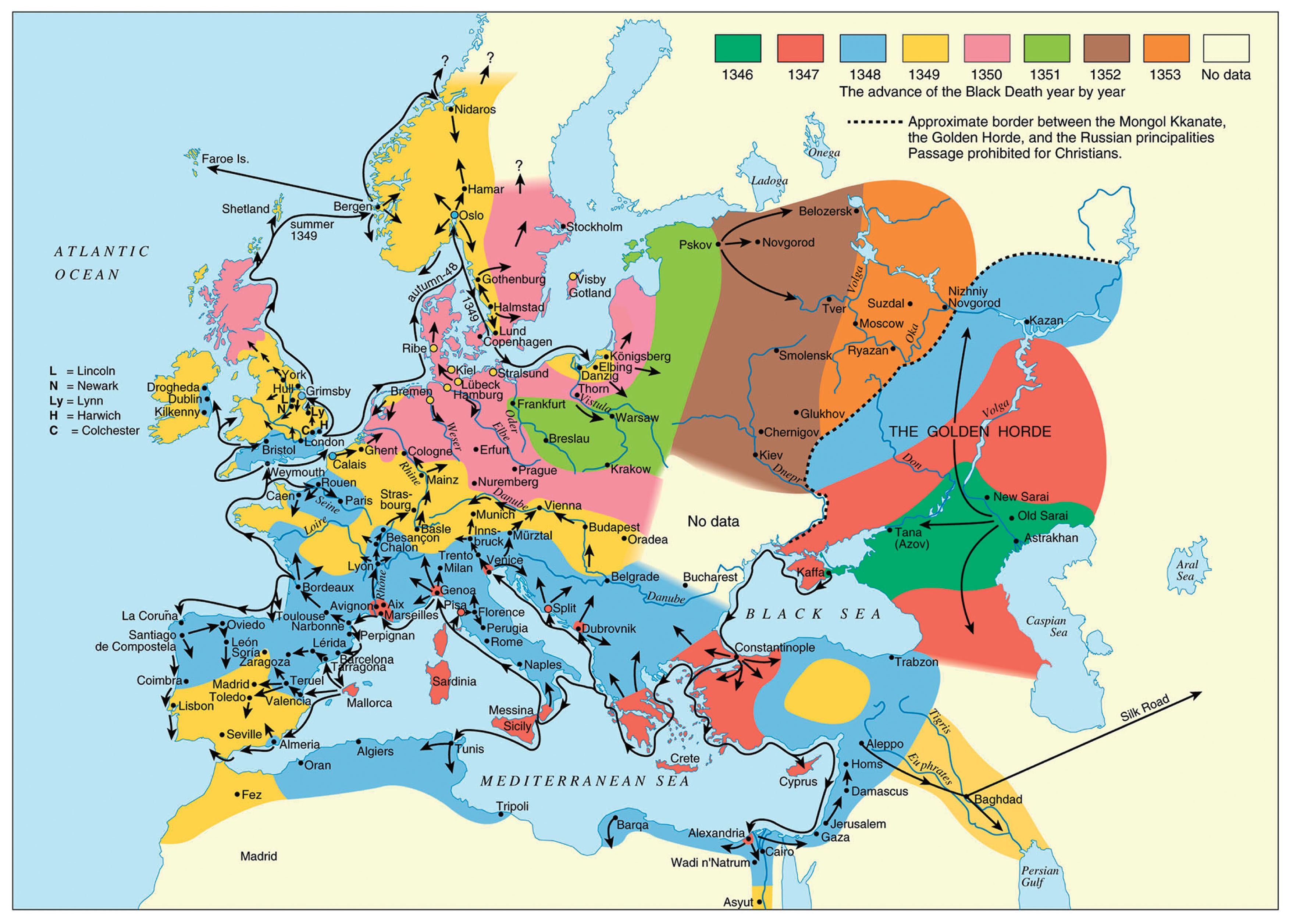
Spread of the Black Death (from the original outbreak to its end), 1346–1353.
This would especially occur in connection with the Italian factory and trading station in the Crimea (Kaffa). This town was situated close to the vast southern Russian plague reservoir among gerbils and susliks, which includes the Astrakhan region (oblast’), that stretches from the northern or north-western coast of the Caspian Sea, to the estuary of River Don in the west in the Rostov region (oblast’), and almost to present-day Volgograd in the north (Fyodorov, 1960; Pollitzer, 1966; Nikolayev, 1972; Ouagrham-Gormley, 2006; Benedictow, 2016, 2017). According to contemporary Russian and Byzantine chroniclers and historians, the Black Death broke out in April 1346 in the lands of the Mongol Kipchak Khanate of the Golden Horde and ravaged their main urban administrative centres of Astrakhan’, Old Sarai and New Sarai (near present-day Volgograd). In 1343, the Mongol-Tartar army drove the Italian merchants, mainly Venetians, out of Tana, their factory at the northern end of the Sea of Azov (present-day city of Azov). They fled to the highly fortified Genoese factory in Kaffa on the Crimea, which was much easier to defend. Kaffa was in its turn besieged, and it was in the autumn of 1346 that the Black Death broke out among the beleaguering army. Later, it broke out in the town until halted by cold winter weather (Benedictow, 2017). In the early spring, a few Genoese and Venetian galleys left Kaffa for their home cities, while the main fleets of galleys remained to fight in order to secure possession of the town, eventually successfully (Henschel, 1842), and returned as usual to their home cities in the autumn, in November.
The galleys that left Kaffa early in the spring were highly probably the source of the contamination of Constantinople in early May 1347. The Venetian galleys reached the island of Crete, and contaminated the important Venetian port town and trading station of Cania (present-day Iraklion), but were, apparently, unable to continue, incapacitated by the Black Death. Venice was contaminated by the return of the main fleet in mid-November 1347, and the presence of the Black Death was not recognized until late March 1348 (Brunetti, 1909).
Two Genoese galleys reached Messina in the second half of June 1347 (Benedictow, 2017) and succeeded in travelling home, spreading the Black Death all the way. They sailed along the secure Genoese lanes from Messina to Trapani, crossed over to Sardinia and used the established network of support and relief ports. In the process, they contaminated Sardinia and Corsica at a time that allowed the development of epidemics there, resulting in the consequent flight of people from the plague to nearby Elba and further across the narrow sound to the important port town of Piombino on the mainland. This occurred in time to allow the development of epidemics also there (Villani, 1728; Dei and di Tura, 1729).
The two Genoese galleys arrived in Genoa in the second half of July, probably after having crossed from Corsica to the south-eastern end of the Genoese territory of Liguria at the Bay of La Spezia and sailing along the coast to Genoa, quite likely also contaminating port towns along this lane. This explains why the Black Death broke out in Marseilles about 1 November (Annales sancti Victoris Massiliensis, 1874), which means that this city of around 15000 inhabitants (Baratier, 1961) must have been contaminated around seven weeks earlier.
The merchant ship(s) from Marseilles that loaded contaminated goods in Genoa’s harbour would usually sail the return stretch of about 400 km to Marseilles, and would, along the sea lane, normally make calls at various port towns for trading. This indicates a time of contamination in the harbour area of Genoa around mid-August, before the outbreak there or just perhaps in an incipient (almost) imperceptible early endemic phase. Because of the slow early phase of plague epidemics, which starts with a 3 week long zoonotic phase in the originally infected rat colony and continues with quite a slow but increasingly dynamic spread between rat colonies and the beginning of spread by people picking up infective rat fleas in their clothing or by local trade and peddling of grain and flour, the outbreak would usually reach a recognizable phase in mid-September. Then, dissemination caused also by refugees fleeing the Black Death would soon begin, until the epidemic waned away with increasingly cold weather and imperceptible spread of infective rat fleas by trade grew in importance.
The mortality caused by the Black Death has been underrated in Italy, although not by contemporary chroniclers and commentators who rather tended to exaggerate, but should, nonetheless, be taken seriously. The available mortality rates in Tuscany and in the Piedmont indicate, in fact, that rates of around 60% were usual. The mortality rate in Florence was about 60%. A recent estimate of the mortality in the 17 main parishes in le Sei Miglia, the rural districts around Lucca, is around 64% (Benedictow, personal communication); the commune of San Gimignano appears to have lost about 56% of its households in the period 1348–1350, corresponding to a population decline of well over 60%, when the usual super-mortality among children and women in relation to adult, predominantly male, householders is taken into account. The city of San Gimignano lost about 66% of its population. Not all urban centres, rural districts or villages were quite so severely ravaged: in the commune of Prato the population decline was apparently about 42.5–45%, eight villages near Susa in the Piedmont apparently lost over 50% of their population, and so on. Taken together, the data indicate a general mortality rate of near or around 60%, similar to France and England (Benedictow, 2017). On the eve of the Black Death, Genoa counted 60000 inhabitants (Pinto, 1996). If the mortality rates in Genoa were of the same order as those registered in Florence, which should be expected, then the population would have been reduced by around 36000 deaths to around 24000 inhabitants in the aftermath of the Black Death.
Even though the catastrophic effects of the Black Death’s arrival in Italy are documented (Benedictow, 2017), no burial sites dating to this event had so far been uncovered in Liguria. Consequently, the discovery of a multiple and simultaneous burial (T34), contemporary to this period, represented a unique opportunity to confirm biologically the presence of plague at that specific time. The burial lies in the cemetery of the 14th-century medieval ‘ospitale’ located on top of Mount San Nicolao (Bracco Massif). The ‘ospitale’ of San Nicolao was used as a rest and recuperation station by traders and travellers (e.g. pilgrims heading to Rome). The successful retrospective diagnosis of the F1 Yersinia pestis-specific antigen in three of four analysed individuals confirms that they did die of plague. The San Nicolao ‘ospitale’ represented a key point into the system of pathways forming the initial part of the Via Francigena in the Italian territory and, as a consequence, was an ideal ground for plague rapid dissemination.
Between 2001 and 2006 excavation campaigns were carried out at the site of San Nicolao di Pietra Colice (Genova), a small rural area located on a plateau on the northern flank of Mount San Nicolao at 792 m.a.s.l. (Benente, 2005, 2008; Benente et al., 2004). A medieval ‘ospitale’ (hospital) along with an annexed small church and graveyard were uncovered. Based on the stratigraphy, it was possible to establish that the site was used between the second half of the 13th century and the late 16th century CE.
The foundation of the ‘ospitale’ has been linked to the consolidation of the power of the Fieschi family in Genoa and its surroundings. Since the 13th century, the Fieschi had promoted an intense donation of goods for the whole community. This action has led to the construction of several churches and annexed hospitals used as facilities for traders and pilgrims heading to Rome and travelling along the two major communication routes of Liguria. These routes formed a network of primary, secondary and tertiary paths linking the north-western part of Italy to Emilia Romagna and upper Tuscany. One route ran down the coast from Genoa through the villages of Sarzana, Brugnato, Sestri Levante and, then, entered Lunigiana (Tuscany). The second route, orthogonal to the coastline, passed through Parma, Piacenza, Sestri Levante, Lavagna and reached Lunigiana (Benente, 2005).
In the graveyard surrounding the small church of San Nicolao di Pietra Colice, a multiple primary burial (T34) containing the skeletal remains of four individuals—a pregnant woman and her fetus and two sub-adults)—was uncovered in 2006. All the remaining burials were single graves. Taphonomy (Duday, 2006; Castex, 2008) allowed the archaeologists to establish that the corpses had been buried simultaneously directly into the ground soil as attested by the persistence of inter-phalangeal ligaments of both hands and feet and the mutual position of the bodies. Stratigraphy dated the burial to the second half of the 14th century.
Anthropologic and paleopathological analysesThe biological profile of the adult individual was determined by using standard anthropological methods that included: (i) the estimation of age at death (Lovejoy et al., 1985; Brooks and Suchey, 1990); (ii) the assessment of female sexual characteristics (Phenice, 1969; Acsádi and Nemeskéri, 1970; Ferembach et al., 1980; Brothwell, 1981; Sutherland and Suchey, 1991); (iii) the calculation of the stature (Trotter and Gleser, 1952; De Mendonça, 2000); (iv) the diagnosis of dental and skeletal abnormalities (Goodman and Rose, 1990; Ortner, 2003).
The sub-adult age at death was established by resorting to the evaluation of the degree of dental mineralization and eruption, the measurement of the diaphyseal length of the long bones and the evaluation of the degree of epiphyseal fusion of the long bones and hip bone (Fazekas and Kósa, 1978; Ubelaker, 1979; Liversidge and Molleson, 2004; AlQahtani, 2008; Schaefer et al., 2009).
During the bio-archaeological investigation, the human remains belonging to the three individuals and a fetus were carefully identified by resorting to an accurate sieving procedure, a process that resulted in a complete reconstruction of their skeletons. All individuals showed an excellent state of preservation (Figure 2a–d).
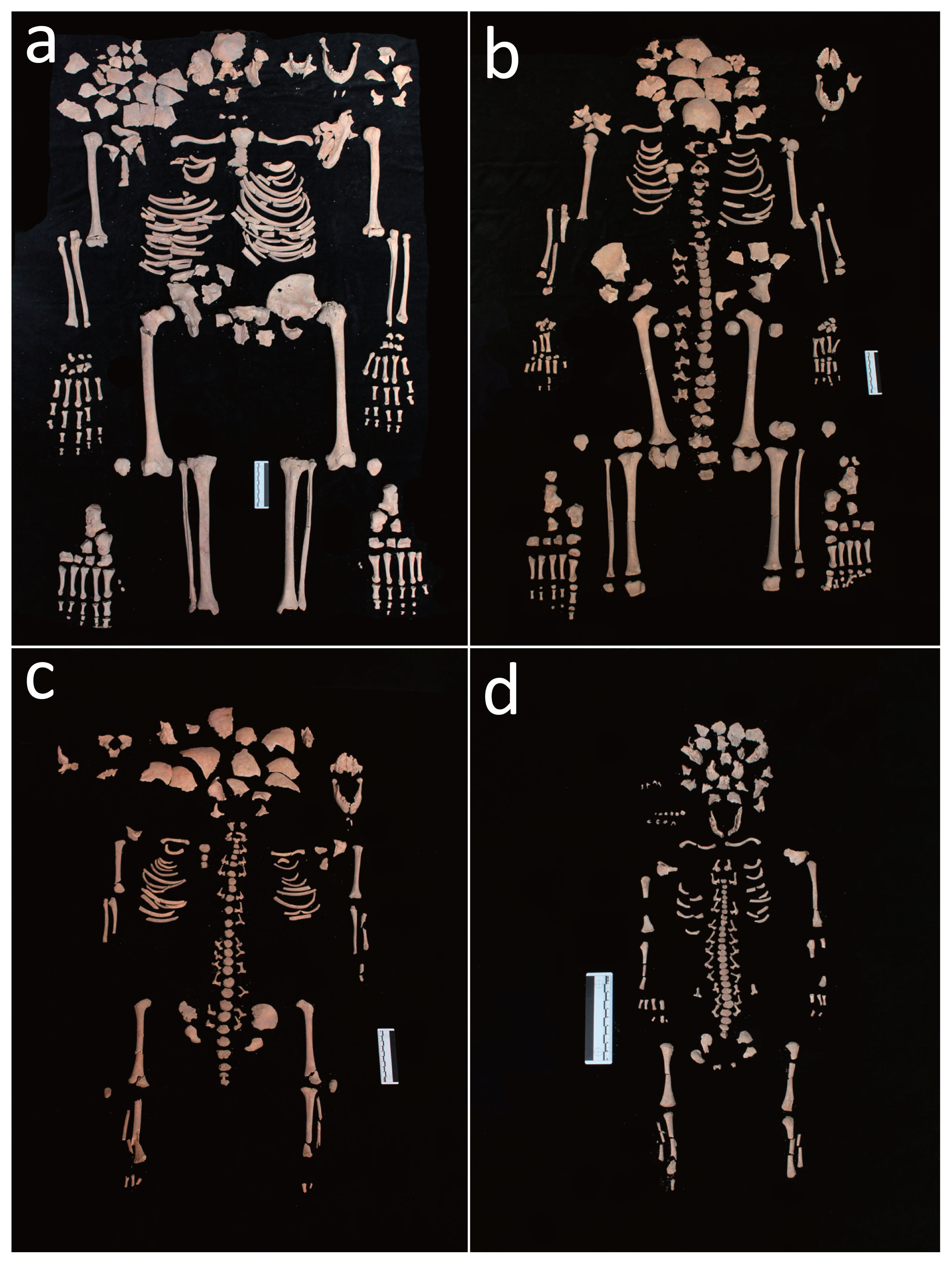
(a) Skeleton of the adult female (T34 US 2614 SN06 ind. A). (b) Skeleton of the 12 year old sub-adult (T34 US 2616 SN06 ind. B). (c) Skeleton of the 3 year old sub-adult (T34 US 2615 SN06 ind. C). (d) Skeleton of the 38–40 week old foetus (T34 US 2617 SN06 ind. D/F).
To detect Y. pestis F1-specific envelope glycoprotein, the Rapid Diagnostic Test (RDT) for plague was employed, an immunochromatographic assay extensively used in bioarchaeological investigations (Bianucci et al., 2007, 2008, 2009; Kacki et al., 2011). Bone samples from the woman and her fetus and from the two sub-adults (Table 1) were analyzed as previously described (Haensch et al., 2010).
| Sample | Sex | Estimated age | Bone | RDT | [AgF1] |
|---|---|---|---|---|---|
| T34 US 2614 SN06 ind. A | Female | 30–39 years | Iliac crest fragment | Positive | 2.5–5 ng/ml |
| T34 US 2617 SN06 ind. D/F | Unknown | 38–40 weeks | Vertebral body | Positive | 2.5–5 ng/ml |
| T34 US 2616 SN06 ind B | Unknown | 12 years | Fragments of vertebral body | Positive | 2.5–5 ng/ml |
| T34 US 2615 SN06 ind. C | Unknown | 3 years | Fragments of vertebral body | Negative | — |
Bones from three of four individuals gave positive Y. pestis F1 signals (Table 1), with concentrations of the antigen ranging between 2.5 and 5 ng/ml, thus confirming that their death was caused by Y. pestis infection.
The female individual (T34, US 2614 SN06, ind. A), who was about 156 cm tall, was aged between 30 and 39 years when she died (Figure 2a). Her stature is consistent with the average height calculated for medieval female population in Northern Italy (Vercellotti et al., 2011).
Paleopathological investigations showed that she had suffered both from dental and skeletal ailments. Linear enamel hypoplasia (LEH), the onset of which had occurred during her childhood around the age of 7, could be appreciated on the incisors (11, 21, 31, 41) and canines (13, 23, 33, 43) (Fédération Dentaire Internationale, 1971). Dental plaque was identified on the lingual side of the anterior teeth (31, 32, 41). Evidence of periodontal disease was seen in the left lower jaw molars (in particular 36) (Figure 3a, b).
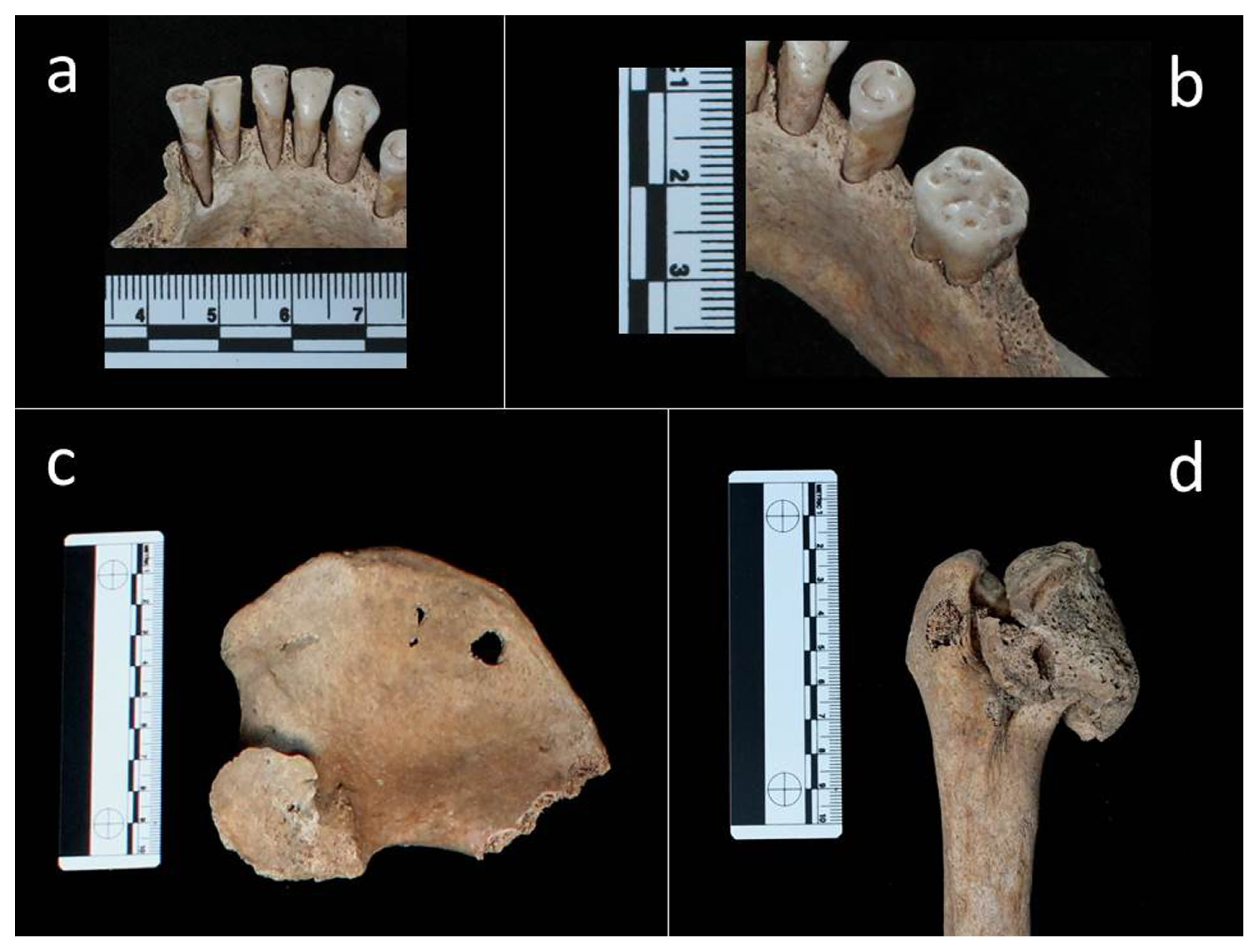
Adult female (T34 US 2614 SN06 ind. A): (a) dental plaque on the lingual side of teeth 31, 32, 41; (b) periodontal disease in tooth 36; (c) abnormal shape of the left acetabulum; (d) abnormal shape and size of left caput femoris.
A unilateral abnormality of the left hip joint was identified. The caput femoris and acetabulum displayed an abnormal shape and size (Figure 3c, d). The head of the femur has lost the normal spherical shape and displayed an oval shape with a ‘mushroom-like’ aspect (Figure 3d). The cortical layer of the left proximal epiphysis showed areas of macroporosity consistent with a loss of bone tissue. The corresponding acetabular surface had lost the normal concave appearance; it showed a hypertrophy of the bone tissue (proliferative) spaced out with extensive bone loss areas (osteolythic) that resulted in marked porosity observable at the hip joint (Figure 3c). Bony proliferation and osteolysis are probably correlated to osteoarthritis, whereas the abnormal direction of the proximal femoral epiphysis is a malformation known as coxa-magna.
This woman, who displayed a congenital hip dysplasia and a unilateral slipped femoral caput, was most likely affected by Legg–Calvé–Perthes disease (Smrcka et al., 2009; Wasterlain and Umbelino, 2013). This disease is originally related to an interruption of the vascular flow usually caused by a traumatic event resulting in an aseptic extensive necrosis of the head of the femur. When revascularization occurs, due to the bony tissue reaction, the femoral head assumes the typical ‘mushroom-like’ shape (Aufderheide and Rodríguez Martín, 1998; Ortner, 2003; Waldron, 2009).
As far as the fetus (T34, US 2617 SN06, ind. D/F) is concerned, it was delivered from the uterine cervix and partially expelled after the mother’s death (Schaefer et al., 2009) (Figure 2d, Figure 4a). This phenomenon, which has seldom been reported in archaeology, is known as post-mortem fetal extrusion or “coffin birth” (Sublimi Saponetti et al., 2013). The decomposition of the mother’s body (e.g. the decomposition of the placenta rich in water, nutrients and enzymes) and the formation of putrefaction gases result in the expulsion of the fetus, which was found in the birth canal.
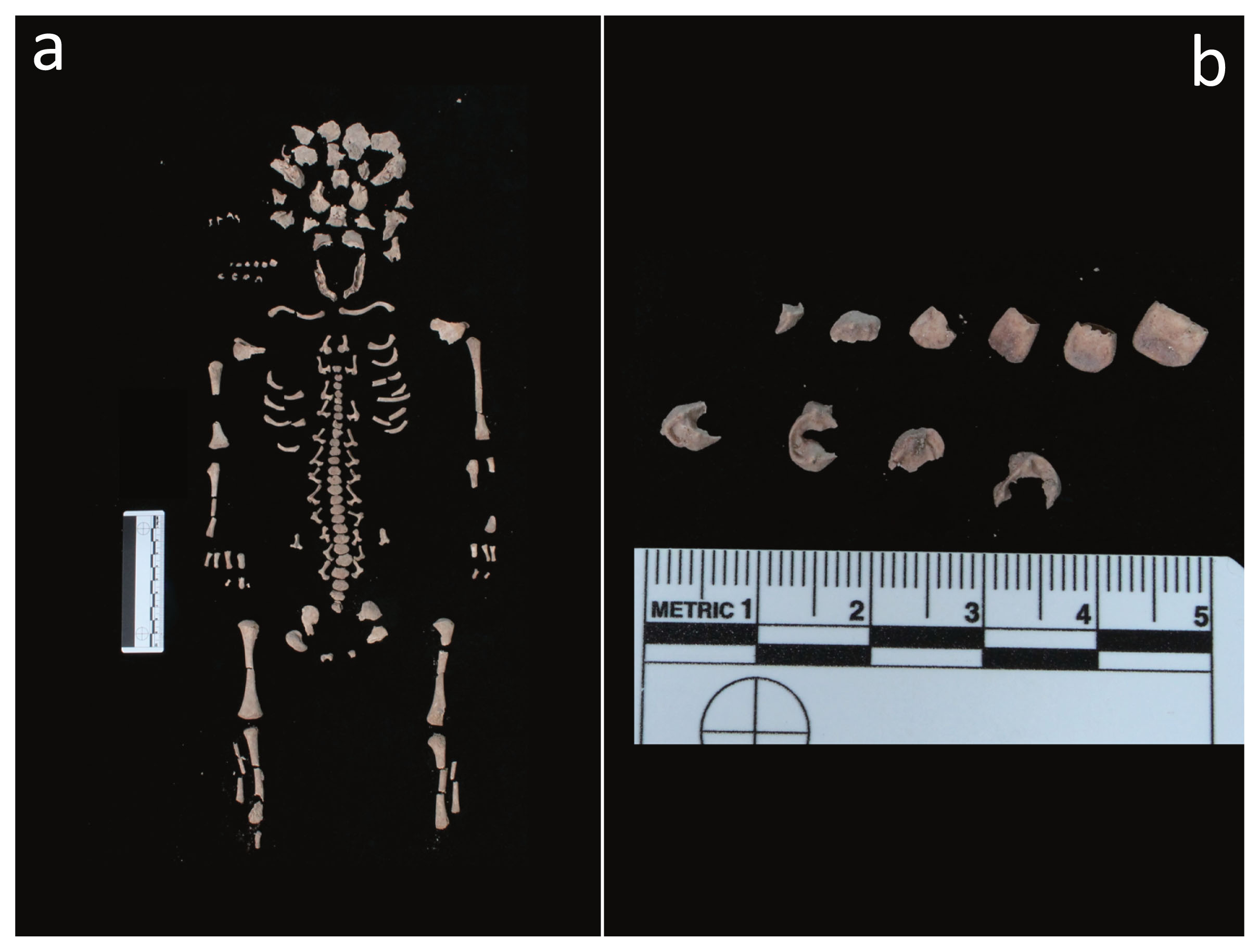
Fetus (T34, US 2617 SN06, ind. D/F): (a) skeleton, general view; (b) deciduous teeth, detail.
Based on dental eruption and on skeletal maturation, it was established that the two sub-adults died aged 3 (T34, US 2615 SN06, ind. C) and 12 years old (T34, US 2616 SN06, ind. B), respectively (Figure 2b, c). The skeleton of the 12 year old sub-adult showed sign of a specific lesions possibly linked to metabolic diseases or nutritional deficiencies. More specifically, the individual displayed cribra orbitalia in the right orbit (Figure 5a) and a marked cortical porosity of the distal femoral metaphysis; the latter is also characterised by the presence of small spicules consistent with bony tissue proliferation (Figure 5b). In infants, a distinction between a normal growth-related bone porosity and bone remodelling due to an inflammatory response consequent to chronic hemorrhage is challenging. Differential diagnosis may include acute inflammation, a common finding in children, or a manifestation of metabolic disease such as scurvy, rickets or anaemia.
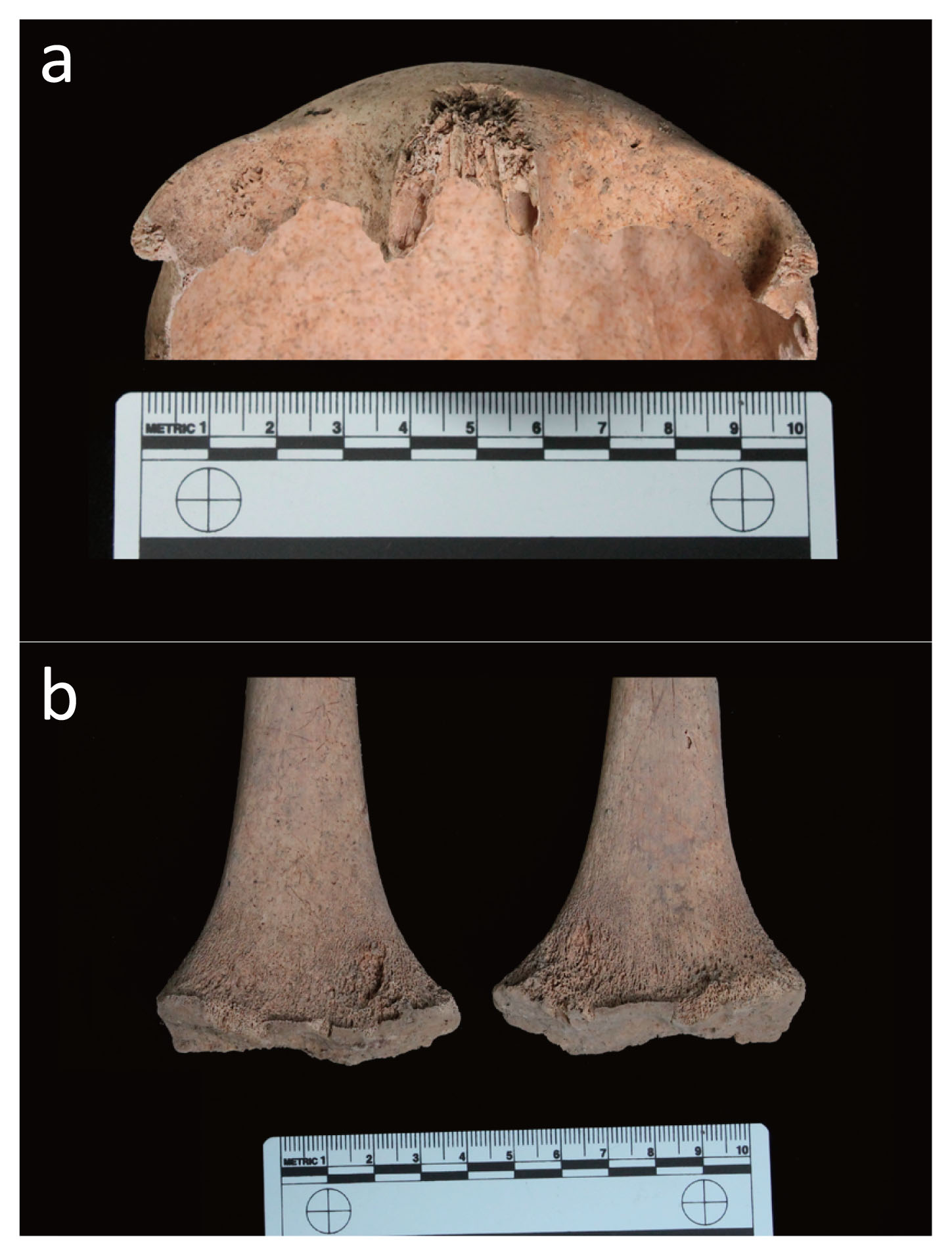
Twelve year old juvenile (T34 US 2616 SN06 ind. B): (a) light cribra orbitalia on the right orbit roof; (b) bilateral spicules and abnormal porosity of the cortical bone of the femoral metaphysis.
Considering the bilateral localization of the lesions, their porosity and the diffused hypertrophy with new bony formation (spicules), scurvy appears to be the best candidate. Due to a prolonged deficiency of vitamin C, which is essential to maintain an adequate immune response to infection, defects in calcium metabolism and normal production of collagen occur. The vitamin C deficiency is generally attributed to poor diet lacking fruit and vegetables and limited subsistence resources (Goodman and Armelagos, 1989; Beaumont et al., 2015).
The results of the immunological investigation provide evidence that the individuals exhumed from multiple grave T34 died of Y. pestis during the Black Death. We thus corroborate previous archaeological and taphonomic evidence with biological findings.
From a paleopathological and a taphonomic perspective, the skeletal remains of the 38–40 week pregnant woman are of particular interest. The woman was affected by Legg–Calvé–Perthes disease, a childhood osteochondrosis of the femoral head (Aufderheide and Rodríguez-Martín, 1998; Smrcka et al., 2009) whose aetiology remains unclear (Hosalkar and Mulpuri, 2012).
As to the causative factors, the potential role of traumatic, genetic, metabolic, nutritional, environmental, hormonal and haematologic origins has been considered and discussed (Smrcka et al., 2009; Herrerín and Garralda, 2012). Following the prevailing theory, the origin is either inflammatory or a traumatic. Approximately 5% of children with transient synovitis of the hip and associated synovial effusion in the joint develop the complication of Perthes disease (Salter, 1999). In the majority of these cases, the onset occurs in 3–10 year old children (average c. 6 years) (Aufderheide and Rodríguez-Martín, 1998; Ortner, 2003; Waldron, 2009). In general, the degree of recovery may vary considerably from good to extremely poor depending on the age of the affected child. If the onset of the disease occurs before 5 years of age, an almost complete recovery may take place; if the onset occurs between the age of 5 and 9 years and more than half of the caput femoris is involved, a discrete recovery can occur. If the pathology manifests after the age of 10, a poor recovery is expected. In younger children, the period of time occurring between the onset of the disease and the skeletal maturity is longer and, therefore, a fine remodelling of the head of the femur may occur. In children aged 10, this period of time is considerably shortened.
Furthermore, taphonomy showed that the woman underwent a “coffin birth” with the fetus being partially extruded due to the build-up of gas pressure within the decomposing body of his/her mother. This phenomenon has been seldom described in the archaeological literature (Sublimi Saponetti et al., 2013; Augias et al., 2015).
The identification of plague victims in the cemeterial area associated to the ‘ospitale’ of San Nicolao proves that the contamination, which had originally started from Genoa’s port area, progressively spread through the principal communication routes. Since the Roman period, there had been a network of routes winding along the Bracco Massif; these paths linked Liguria, Tuscany and Emilia Romagna and were widely used both for trades and by the army. The centre of this complex network—known as a ‘fishbone’ system of paths—was Mount San Nicolao, whose name in the Middle Ages was Pietra Corice/Colice. Originally, this mountain pass was part of the Via Aurelia, the route that linked Liguria to Etruria. The ancient Peutinger’s Table (medieval copy, 12th–13th century CE) shows all the routes of the Roman Empire, including the Via Aurelia (Prontera, 2003). After Luni and shortly before Moneglia (Ad Moneliam), it mentions a station named Alpe Pennino, which should correspond to the area of Pietra Colice.
This ‘fishbone’ system of routes was characterized by a horizontal path from Sestri Levante to Brugnato and a series of vertical paths, which crossed the main route, and then climbed down to Val Petronio and headed to the Val Padana. One route started from San Lazzaro (Casarza Ligure), climbed the Bracco and headed east to Mattarana, Carrodano and Borghetto. Then the route went down at Brugnato and headed to Lunigiana. From Lunigiana, Lucca was easily reached, thus entering the Francigen route. Another route, which came from Levanto and flanked Mount Sant’Agata, also led to San Nicolao.
From San Nicolao, the path could either go into the Mattarana direction or come down to Val Petronio. By-passing the Baracchino Pass or the Bocche di Vasca Pass, it was possible to reach another major route, which went through Castiglione Chiavarese, San Pietro Vara, Varese Ligure and headed to the Cento Croci Pass and to Val di Taro in Emilia.
Such a system of routes was already mentioned in a document written in 774 CE. The original document has been lost but a copy dating to the 12th–13th century exists (Garbarino, 1992; Andenna, 2001). The document mentions that Charlemagne gave lands to the monastery of San Colombano di Bobbio (Piacenza) and that one of the ‘public’ routes that could be used to reach the monastery passed through Pietra Colice. Since the term ‘public routes’ was used, this implies that these routes were a property of the Carolingian Empire, which, in turn, had reused the old itinerary used during the Roman Empire.
This analysis indicates that Liguria was covered by epidemic forces of the Black Death that fanned out from Genoa westwards towards France and northwards into and across Piedmont and Lombardy, and north-eastwards deep into northern Emilia via Bobbio 50 km north of Genoa and also, according to the chronicler (Henschel, 1842), 40 km further to Piacenza, his home city. The point of departure for the latter movement would the Black Death’s early spread out of Genoa eastwards and, next, south-eastwards along the Liguria region where it at some point may have met the plague that was spreading north-westwards from La Spezia.
While plague was heading from Liguria towards Lucca’s countryside, via the system of routes forming the Francigen Route, several cities and towns in Tuscany were acting simultaneously as independent epicentres.
The pattern of outbreaks in the northerly parts of Tuscany from the early spring of 1348 shows that Pisa too was contaminated in the autumn of 1347 and established as an original epicentre of the Black Death. This was most likely associated with the grain trade in which this big commercial centre was deeply engaged. It was the main supplier of northern Tuscany including important cities such as Florence, Lucca, Volterra and Pistoia and a densely populated wide hinterland with towns, villages and hamlets. The Volterrans also co-operated with the Florentines to purchase provisions of grain from Calabria, Sicily, Sardinia and North Africa (Maffei, 1887; Battistini, 1916).
Another epicentre of the Black Death was Reggio Calabria; this epicentre was established in the autumn of 1347, when Messinese fled from the Black Death just across the narrow strait to the mainland (Michele da Piazza, 1865). The likelihood that grain from Calabria, Sicily and Sardinia would carry rat stowaways and be heavily contaminated by infected rat fleas would be very high.
The sailing ships most used for transportation of grain and raw materials, which could be acquired when the harvest was over, the so-called round ships or ‘nefs,’ would not return until quite late in the autumn, in October or November. At this time, the weather was chilly and becoming colder. Therefore, given the unfavourable environmental conditions, the inherent development of plague disease in the form of enzootic and epizootic developments in the rat colonies and release of infected and infective fleas from dying rats would have been suppressed or strongly limited. The smouldering processes of plague in the rat colonies would be (almost) unobservable and unrecognized. In the following months, contaminated grain, flour and other goods would be spread over largish distances and to numerous urban centres and townships.
This analysis and social and economic premises predict that with the arrival of warmer spring weather plague would break out almost simultaneously in many localities. These localities that might apparently seem unconnected, were, in reality, connected to common sources of infection introduced in the cold season (Benedictow, 2017). This prediction is corroborated. It is, though, useful to take into account that it took over two weeks longer in big cities than in small towns from the incipient endemic outbreaks to the presence of the Black Death being recognized by the chronicle-producing upper classes on whose account we rely upon for dating (Benedictow, 2017).
Outbreaks of the Black Death were recognized in March in Lucca and in the surrounding countryside of le Sei Miglia and Florence, in late March or early April in Pistoia, in (late March or) early April in Perugia (in Umbria), in April Pisa, Siena, Orvieto (in Umbria, there was huge mortality by 1 May), and in Bologna and as far away as Naples at the end of March—a big city also exposed to importation of contaminated grain and flour from Calabria and Sicily (see Chronicon Estense [AA.VV., 1729]).
The theory of the epidemiology and dynamics of spread of the first phase of the Black Death in Italy from the original mainland epicentres can now be tested, using Tuscany with Umbria and the southern region Emilia Romagna, regions for which superior data exists. The standard assumption on the pace of development from the introduction of plague infection in a local rat colony to the outbreak of plague and the recognition of its presence by the chronicle-producing upper classes is 6–8 weeks, depending on the size of the community. Clearly, the temporal pattern of these outbreaks of plague from Pisa, Lucca and le Sei Miglia, Pistoia, Florence and Bologna is incompatible with serial spread by inter-human cross-infection or by refugees from these plague epidemics (Benedictow, 2017)
The outbreaks in Tuscany are for all practical purposes contemporaneous. This pattern is only compatible with rat-flea-borne dissemination and transmission of plague by commercial transportation of grain and flour during the months of late autumn and winter. The contamination must have arrived at these locations and started spreading slowly and enzootically in the rat colonies at points varying by at least several weeks or frequently by months.
The epizootic and epidemiological potential would, however, be activated at about the same time with the advent of warmer spring weather. The temporal pattern of outbreaks constitutes, therefore, proof of the basic epidemiological structures and dynamics of the first phase of plague and their crucial links with the grain trade. This pattern is just as clear in northern Germany where Hanseatic ships returned in the late autumn of 1349. The Black Death briefly revealed its frightening presence in some of the cities (e.g. Lübeck) but was suppressed by cold wintry weather. However, the Black Death returned with a vengeance, and broke out quite contemporaneously in numerous towns and cities in large parts of northern Germany, when spring arrived with warmer weather (Benedictow, 2017). This pattern can also be seen in the study of plague epidemics in the Netherlands in the period 1450–1668 (Noordegraaf and Valk, 1996).
We are indebted to Minoarisoa Rajerison (Pasteur Institute of Madagascar, Antananarivo, Madagascar) for providing the dipstick assay. We are grateful to Dr Fabrizio Benente (MUSEL—Museo Archeologico e della Città di Sestri Levante), to the Soprintendenza Archeologia Liguria for the authorization to analyse the samples, and to Dr Elpis Samantà for her collaboration.